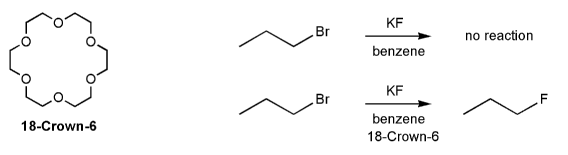
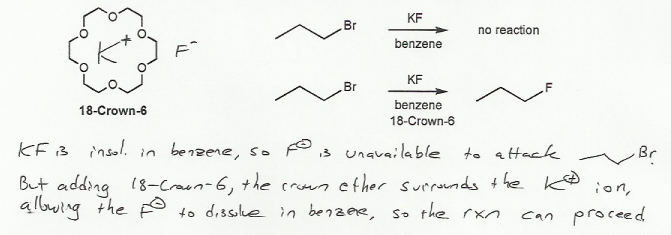
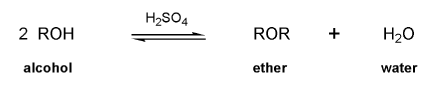
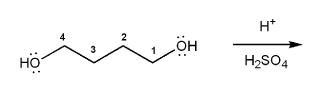
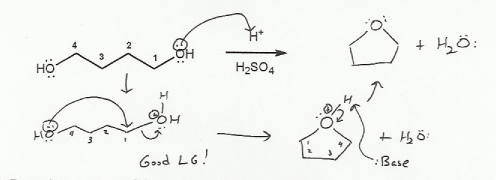
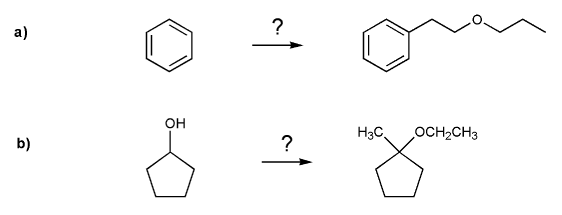
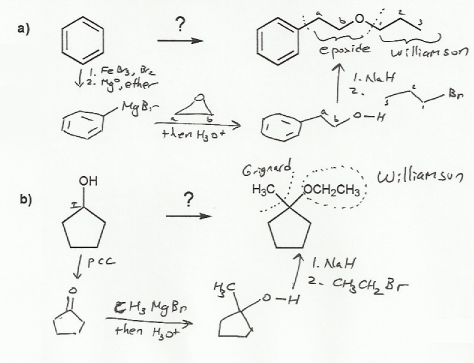
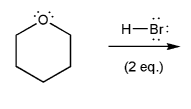
Textbook: Carey and Giuliano 8th Ed. (2010)
Chapter 16: Ethers, Epoxides, and Sulfides
Practice Problems and Mendel Sets
Individual Problems
Mendel Sets
Textbook and Chapter: Carey and Giuliano 8th Ed. (2010), Chapter 16
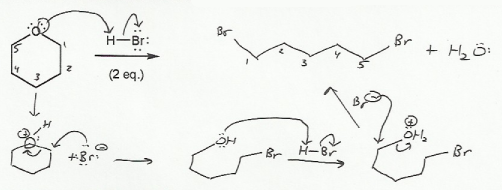
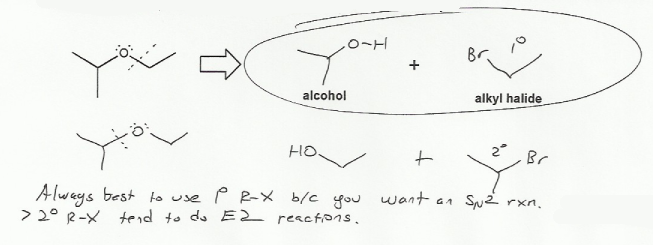
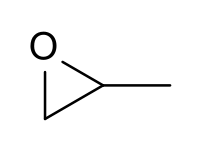
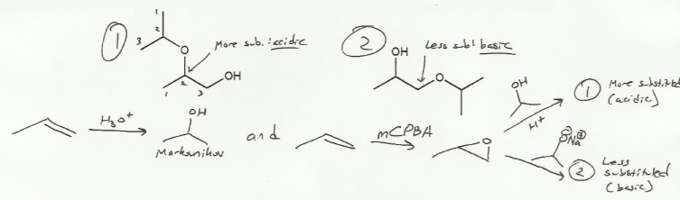
Keywords: epoxides, Williamson ether synthesis
Description: Covers the major topics dealing with ethers and epoxides:
Total Problems: 7