

Show a mechanism for the acid-catalyzed cyclization (condensation) of 1,4-butanediol.
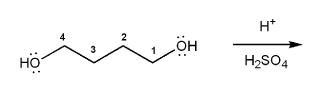
Rank the following compounds in order of decreasing boiling point.
Also, make a guess about their relative solubilities in water. Explain your reasoning.
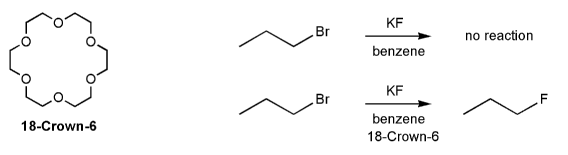
When propyl bromine is treated with KF in benzene no reaction takes place. But when the crown ether 18-Crown-6 is added to the reaction mixture the desired propyl fluoride product is produced. Explain.
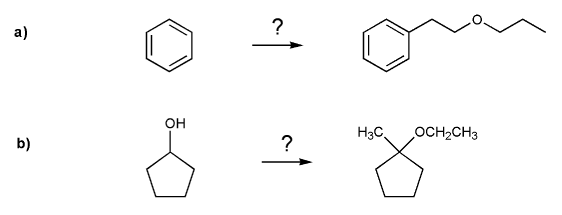
Show how each compound can be prepared from the indicated starting material.
All carbon sources must contain three carbons or less.
Write out a mechanism for the reaction below using curved arrows. Be sure to include formal charges.
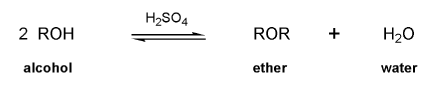
The acid-catalyzed condensation of alcohols to form ethers is reversable; ethers can be hydrolyzed back to alcohols. How can the direction of this equilibrium be controlled to preferentially form ethers?
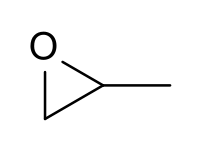
Show how to prepare each compound starting from propylene oxide.

(Propylene oxide image below courtesy of Wikipedia.)
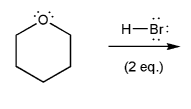
Show two ways to prepare the ether below from a combination of an alcohol and an alkyl halide via the Williamson ether synthesis.
Is one way better than the other? Why?
