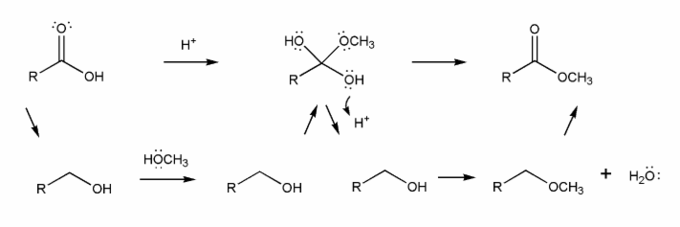
The overall mechanism for Fischer esterification is shown below. This isn't a real mechanism, just an outline.
Methanol (the nucleophile) attacks the carbonyl carbon, forming a tetrahedral intermediate, which then loses a water to reform the carbonyl. This mechanism is called nucleophilic acyl substitution.
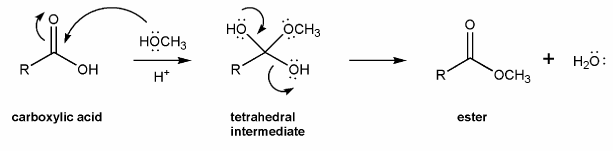
Use curved arrows to draw a full mechanism for this reaction. I've included structures for you to use as a guide.
This reaction takes place under acidic conditions, so the mechanism you draw will be similar to those in problem 706.