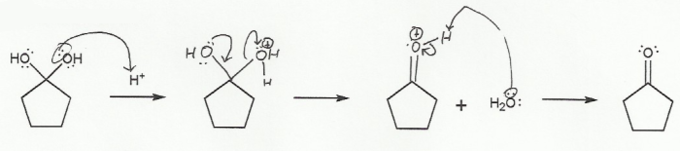
The interconversion between a carbonyl (sp2 carbon) and a tetrahedral intermediate (sp3 carbon) is the most common mechanism you will encounter in second semester organic chemistry.
You should be familiar drawing it under both acidic (this problem) and basic (problem 705) conditions.
In a), the carbonyl "goes up" to form a tetrahedral intermediate.
In b), an oxygen "comes back down" to reform the carbonyl and kick off a leaving group.
You will see this "up, down, kick" pattern in most mechanisms that involve attack at the carbonyl carbon, which is most of the reactions in second semester orgo!
a) Carbonyl to Hydrate (acidic) "UP"
Because this reaction takes place under acidic conditions, the carbonyl must protonate before the nucleophile attacks, to prevent oxygen from ever being negative (ROH instead of RO-).
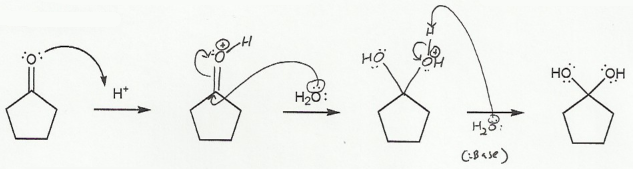
b) Hydrate to Carbonyl (acidic) "DOWN"
The leaving group must protonate before it leaves, so it doesn't leave as a negative molecule (H2O instead of HO-).