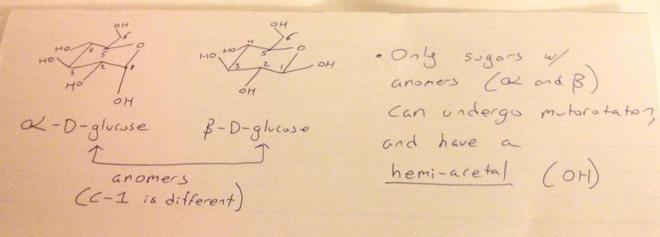
What types of sugars can undergo mutarotation?
Submitted by Shruti6476109
Its like I'm having difficulty in identifying which compounds will show mutarotaion and which won't. Which compounds will show mutarotation in various solvents? Eg- Which of these show mutarotation? --> D-Glucose, L-Glucose, Sucrose, all. Eg Which of these will show mutaroation in aq. solution? --> Glycogen, Cellulose, Sucrose, Maltose