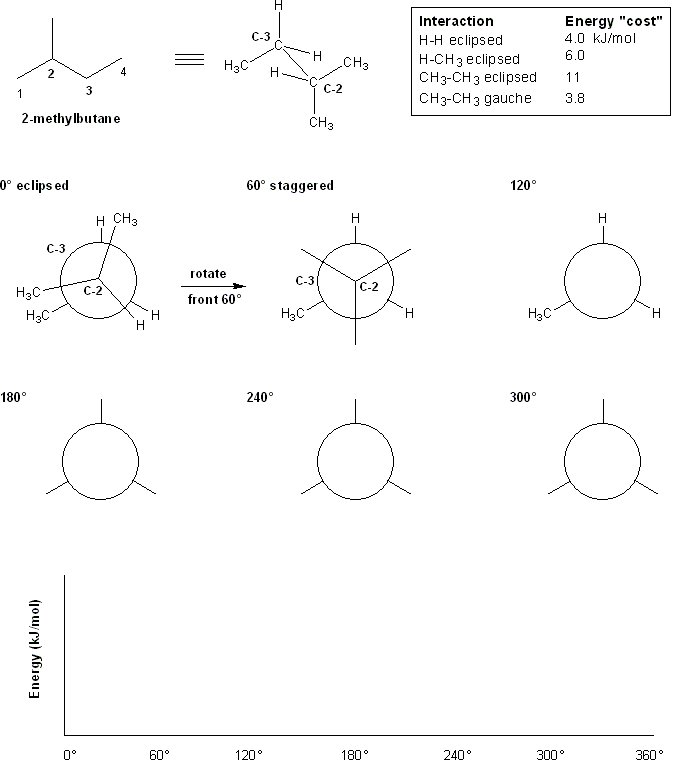
Let's perform conformational analysis on 2-methylbutane along the C2-C3 bond. We'll use the energy chart given below.
First, draw out the Newman projections along the C2-C3 bond, rotating the front carbon (C-2) by 60 degrees clockwise each time while keeping the back carbon (C-3) stationary.
According to the table above, how much energy does each conformation "cost?"
Second, make a plot of the total energy value for each Newman projection versus its dihedral angle.