This week you’re going to do a William ether synthesis, which is covered in chapter 16 of Carey (section 16.6 of the 7th edition). Your instructor will give you a handout with pretty detailed instructions. I’m going to go over the theory first, and then I will show you what to expect.
Alcohols are poor nucleophiles.
Alcohols are pretty lousy nucleophiles. But we can make them more nucleophilic by using a base to rip off a proton and give the alcohol a negative charge. (A deprotonated alcohol is called an alkoxide). The common bases used for this are sodium hydroxide (NaOH) and sodium hydride (NaH).
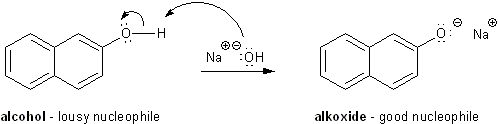
Bases make alcohols better nucleophiles
Now that we have a decent nucleophile, we can react the alkoxide with an electrophile such as an alkyl halide in an SN2 reaction to make an ether.
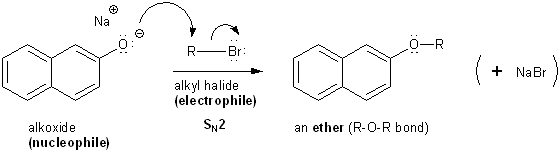
Alkoxides react with alkyl halides to form ethers
This two-step process is called the Williamson ether synthesis.
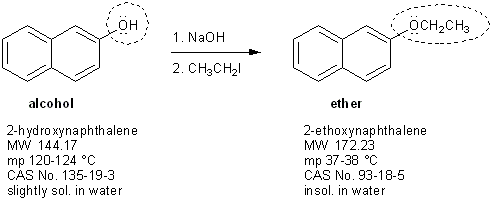
Williamson ether synthesis is two steps
Quick Procedure
You’re going to add ~5 mL of methanol, two boiling stones, and your starting materials (2-hydroxynaphthalene, sodium hydroxide, and ethyl iodide) into a 50-mL round bottom flask and reflux it for about 30 minutes. Be sure to save a little bit of the original starting material mixture to use for a TLC analysis later on!
Afterwards, let the reaction cool and transfer it to a sep funnel to do an extraction with water and ether. Your product is organic soluble and will be in the ether layer, which will be on top (you will discard the aqueous layer, which should contain unreacted starting material).
(Extraction review: www.mendelset.com/articles/685/extraction-and-determination-distribution-coefficient-kd )
Save a little bit of your ether layer for TLC analysis.
Then you will let the ether evaporate over a steam bath, which should yield your crude 2-ethoxynaphthalene product.
Finally, you will do a recrystallization using ethanol to purify your product.
(Recrystallization review: www.mendelset.com/articles/680/preparation-recrystallization-acetanilide )
TLC Analysis and Melting Point
While you’re boiling off the ether layer you’ll run a TLC plate. Your product (2-ethoxynaphthalene) is an ether and can’t hydrogen bond, so it will be less polar than your starting material (2-hydroxynaphthalene). Because of this, you can expect your product to have a higher Rf value than your starting material.
Your TLC will have three spots. One will be your pure starting material, which should be a low riding spot. You will also spot your original reaction mixture, which should contain mostly starting material but may show the presence of a little bit of product. Your third spot will be the ether layer from the sep funnel. It will contain mostly product, but should contain a little bit of starting material.
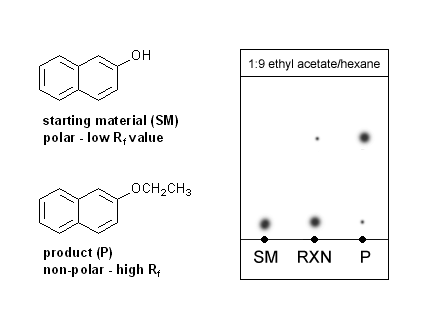
TLC of alcohol starting material and ether product
(TLC review: www.mendelset.com/articles/683/thin-layer-chromatography-tlc )
After your recrystallization is complete and your product is somewhat dry you will take a melting point. The literature value for the melting point of 2-ethoxynaphthalene is 38-39 ºC. They also want you to take a TLC of your purified product. It should look the same as the TLC of your ether layer, but probably won’t have any starting material present.
(Melting point review: www.mendelset.com/articles/676/melting-point )
Calculating Percent Yield
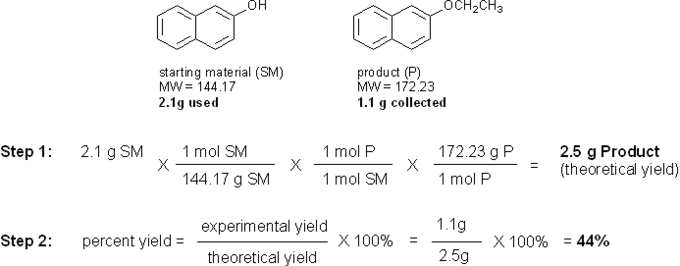
The limiting reagent in this reaction is 2-hydroxynaphthalene, so that is what you will use to calculate the theoretical yield. Let’s say you used 2.1 g of 2-hydroxynaphthalene and collected 1.1 g of pure 2-ethoxynaphthalene product after recrystallization. Your calculations would look something like this:
Calculating percent yield
Spectral Analysis (NMR and IR)
Something you should always include in your lab report is a comparison of the IR and NMR spectra of your products and starting materials.
Your starting material was an alcohol, so should show a hydroxyl peak ( –OH) around ~3,400 cm-1 on its IR spectrum. Your product is an ether and so does not contain a hydroxyl group, and this peak will be absent on its IR.
The only real difference in the 1H NMR spectra will be the presence of two ethyl group signals in your product. These peaks will be absent in your starting material.
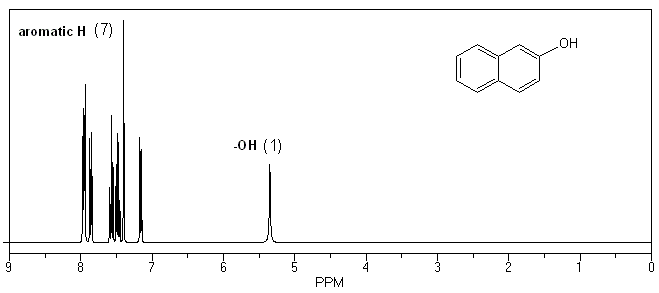
1H NMR of 2-hydroxynaphthalene
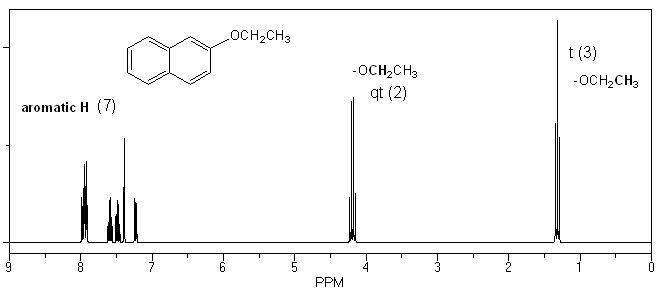
1H NMR of 2-ethoxynaphthalene
That’s it for this week.
