Dehydration of alcohols may result in alkenes.
See:Acid Catalyzed Dehydration of Alcohols
Elimination on an alkane produces alkenes. So, it is just a matter of knowing how elimination occurs.
You may hear alkenes referred to as olefins. They mean the same thing.
When a proton is picked off an alkane, the electrons collapse into the alkane. The process removes a substituent off the alkane to form the alkene. This is an E2 elimination.
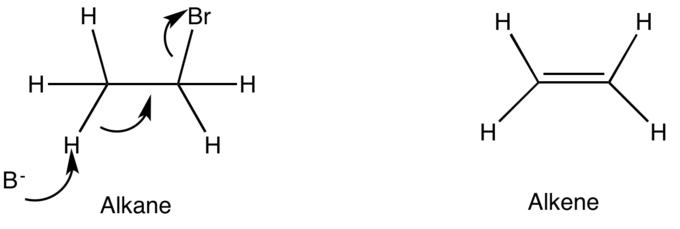
E2 removes hydrogen and the leaving group (LG) at the same time.
This elimination must occur anti-coplanar. Do NOT draw E2 as syn-coplanar.

In E1 reactions, a carbocation is produced before the elimination step. The LG is removed followed by the removal of a hydrogen. This occurs in two steps

Zaitsev’s rule simply means that the more substituted carbon will lose a hydrogen. Does this rule always apply? No, you can treat an alkane with a bulky base that may result in the hydrogen being removed on the less substituted carbon. This is known as Hofmann’s rule
In general, go with Zaitsev’s rule. You should only see Hofmann’s rule apply for specific cases in your studies.

