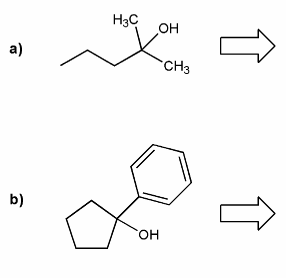
Show how each alcohol can be prepared from a combination of a carbonyl and a Grignard reagent.
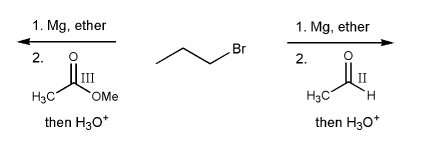
In your own words, what is the major difference in the addition of a Grignard reagent to an oxidation state III carbonyl (ester/acid chloride) versus an oxidation state II carbonyl? (aldehyde/ketone)
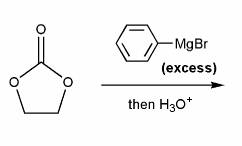
Draw out the mechanism for the addition of excess phenyl Grignard to the carbonyl compound below.
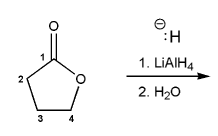
Show a mechanism for the reduction of butyrolactone using LiAlH4.
Carbonyls are in equilibrium with their hydrate forms. This equilibrium happens in both acid and base.
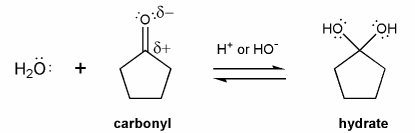
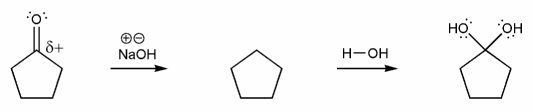
Let's go through this equilibrium under basic conditions. Draw a mechanism using curved arrows for each reaction below.
Remember that under basic conditions, most species are either neutral or negatively charged, and rarely positively charged. So your structures will contain either ROH or RO-, but not ROH2+.
a) Carbonyl to Hydrate
Notice that no oxygen is ever positive during these basic mechanisms (always negative or neutral).
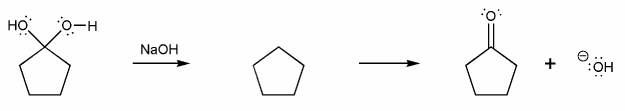
b) Hydrate to Carbonyl
Carbonyls are in equilibrium with their hydrate forms. This equilibrium happens in both acid and base.
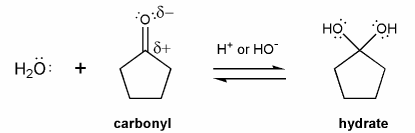
Let's go through this equilibrium under acidic conditions. Draw a mechanism using curved arrows for each reaction below.
Remember that under acidic conditions, most species are either neutral or positively charged, and rarely negatively charged. So your structures will contain either ROH or ROH2+, but not RO-.
a) Carbonyl to Hydrate (acidic)
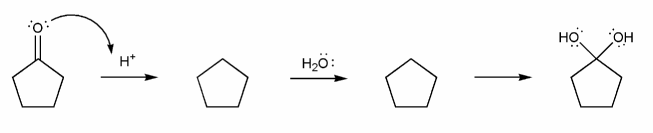
b) Hydrate to Carbonyl (acidic)
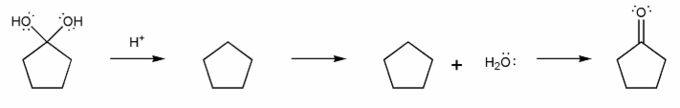
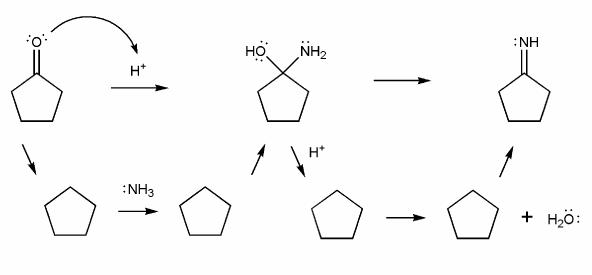
The overall mechanism for imine formation is shown below. (This isn't a real mechanism, just an outline)
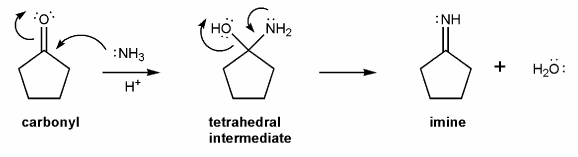
Use curved arrows to draw the full mechanism for imine formation under acidic conditions. (I've added outlines of the intermediate structures for you to use as a guide). This mechanism is similar to that in problem 706 (carbonyl hydrate equilibria).
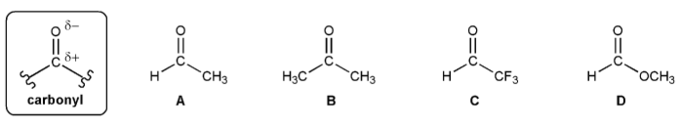
Rank the carbonyls A-D below in order of decreasing electrophilicity (reactivity with nucleophiles).
(1 = Most reactive). Explain your reasoning.
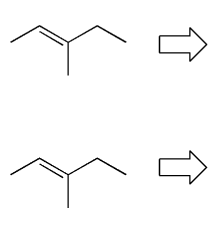
Show two ways of preparing the alkene below via the Wittig reaction starting from triphenyl phosphine (PPh3).
Is one route better than the other? Why?
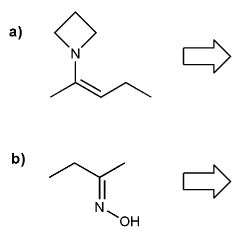
Show what combination of amine and carbonyl would result in each imine or enamine.
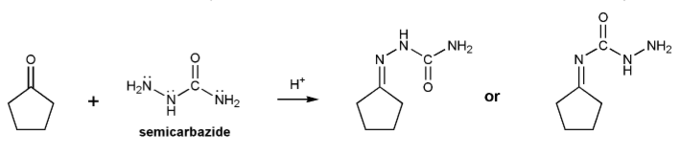
When a carbonyl is treated with semicarbazide under acidic conditions an "imine" is produced called a semicarbazone.
Which of the two products below is the correct structure for a semicarbazone? Explain.
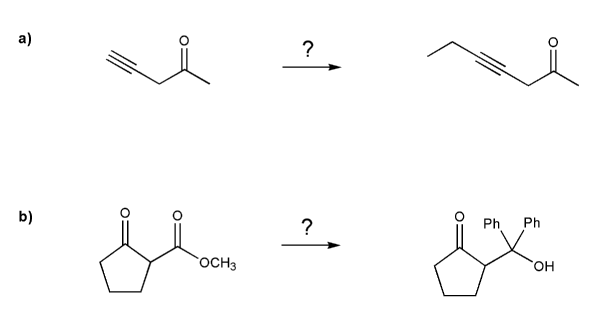
Complete each synthesis below. All carbon sources must come from alkenes.
Each synthesis will involve protecting groups.
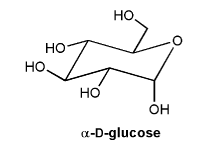
α-D-glucose is shown below.
a) Is α-D-glucose an acetal, hemiacetal, ketal, or hemiketal?
b) Draw the carbonyl form of α-D-glucose.