

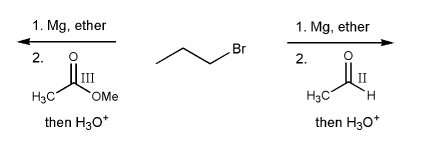
In your own words, what is the major difference in the addition of a Grignard reagent to an oxidation state III carbonyl (ester/acid chloride) versus an oxidation state II carbonyl? (aldehyde/ketone)
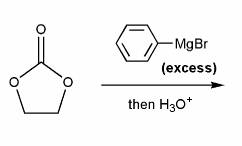
Draw out the mechanism for the addition of excess phenyl Grignard to the carbonyl compound below.
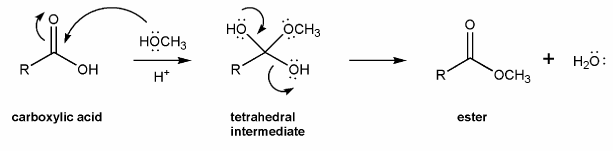
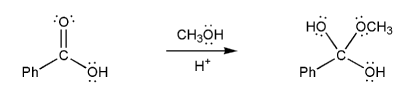
The overall mechanism for Fischer esterification is shown below. This isn't a real mechanism, just an outline.
Methanol (the nucleophile) attacks the carbonyl carbon, forming a tetrahedral intermediate, which then loses a water to reform the carbonyl. This mechanism is called nucleophilic acyl substitution.
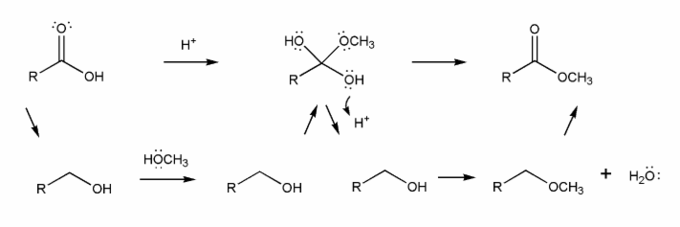
Use curved arrows to draw a full mechanism for this reaction. I've included structures for you to use as a guide.
This reaction takes place under acidic conditions, so the mechanism you draw will be similar to those in problem 706.
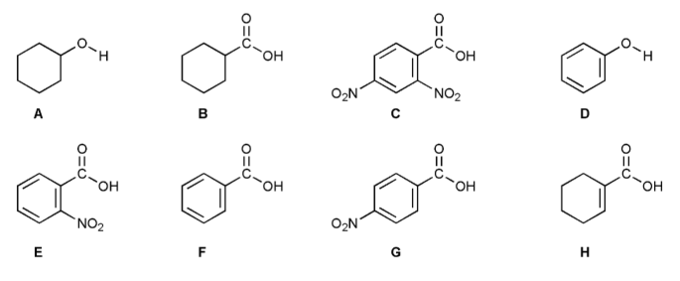
Rank each of the eight compounds A through H below in order of decreasing acidity (1 = most acidic).
Don't get intimidated! What are the differences between these compounds?
Consider electron withdrawing groups (EWG), resonance, hybridization, and the functional group of the acidic proton.
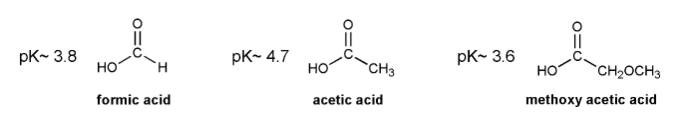
Base your answers to the three problems below on your knowledge of electron donating groups and electron withdrawing groups (EDG and EWG).
a) Based on the pKa's listed below, Is formic acid more or less acidic than acetic acid? Propose an explanation why.
b) If acetic acid were added to a pH = 4.7 buffer solution, what percentage of it would be in its acetate (conjugate base) form?
c) Methoxy (-OCH3) is usually considered an EDG. But based on the pKa of methoxy acetic acid, do you think this is always the case? Explain.
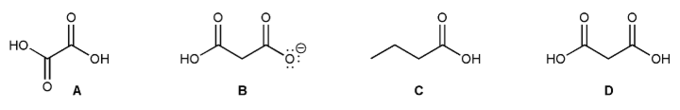
Rank each of the four compounds below in order of decreasing acidity (1 = most acidic).
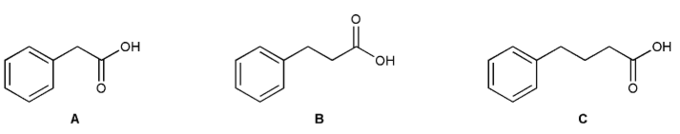
Show how to prepare each compound from vinyl benzene.
How would you prepare the methyl ester of each compound?
Use curved arrows to show the formation of the tetrahedral intermediate of a Fischer esterification reaction (shown below). There are three steps in total.
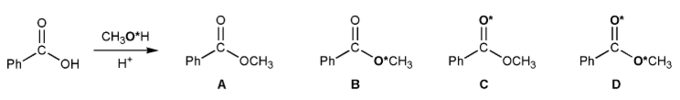
A chemist carried out a Fischer esterification using methanol that was isotopically labeled with 18O (indicated with an asterisk).
Which one of the esters below (A-D) was formed?
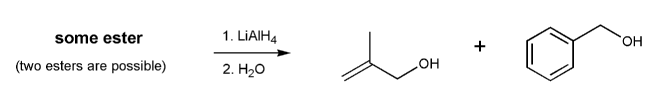
Show two esters that would yield the two alcohols below after treatment with lithium aluminum hydride.
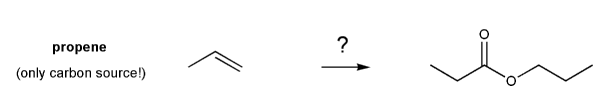
Show how the ester below can be prepared from propene.
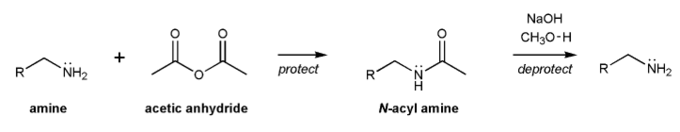
The acyl group is a protecting group for amines. Amines can be acylated using acetic anhydride, and deacylated with base.
Propose a mechanism for each reaction.
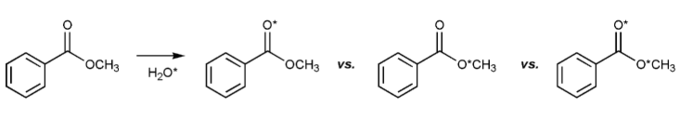
The ester below was dissolved in a solution of water, a small amount of which was isotopically labeled with O-18, denoted with an asterisk.
After a few hours, some isotopically labeled oxygen was found in the ester. Where was it found in the ester? Can you explain why?
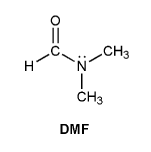
N,N-dimethylformamide (DMF) is shown below. Based on its structure, you might expect to see only one -CH3 signal in the 1H NMR spectrum. But instead DMF shows two different -CH3 signals. Explain.
Show how each ketone below can be prepared from sodium cyanide and either ethylene or propene.
You may also use methyl Grignard and ethylene oxide.
