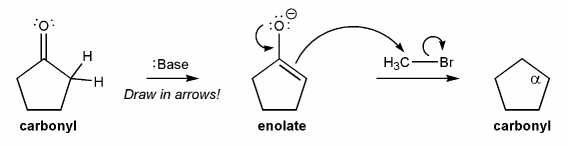
Enolates are formed from carbonyls by adding a strong base, such as lithium diisopropyl amide (LDA), to deprotonate the alpha position. The enolate can then act as a nucleophile and attack an electrophile (such as an alkyl halide), to form a new bond at the alpha position. This is called a carbonyl alpha substitution reaction.
Let's go through the mechanism of how enolates are formed and how they react with electrophiles.
Draw in the curved arrows to show the formation of the enolate (middle compound), and draw the structure of the carbonyl product (right compound)