Mendel Set 2677
Solutions can be seen at mendelset.com/ms/2677
Description: everything
Total Problems: 40
-
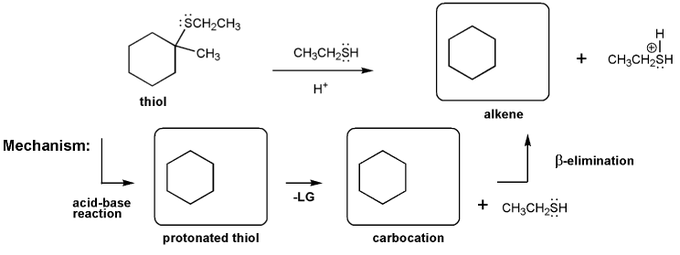
Problem # 519
Let's work through an elimination reaction. Draw the structures for each of the species in the three boxes below (protonated thiol, carbocation, and alkene). Also draw curved arrows to show electron movement.
-
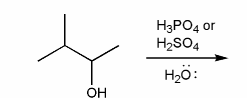
Problem # 341
Predict the product(s) of the reaction below, and used curved arrows to show a mechanism.
-
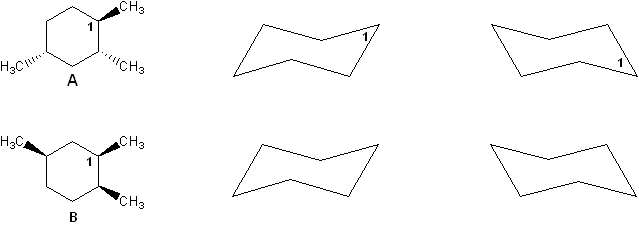
Problem # 318
Two stereoisomers of trimethylcyclohexane are shown below (compounds A and B). Compare cyclohexane chair forms to determine which isomer has a lower heat of combustion. Explain your reasoning.
-
Problem # 319
For a molecule to undergo an E2 reaction, the leaving group and the beta-proton must be in an anti-coplanar conformation (one atom straight up, the other straight down). Based on this, which compound undergoes E2 reaction with KOtBu faster? Why?
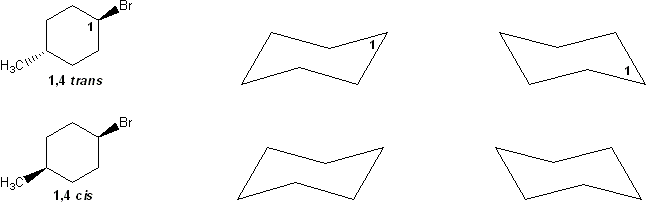
-
Problem # 335
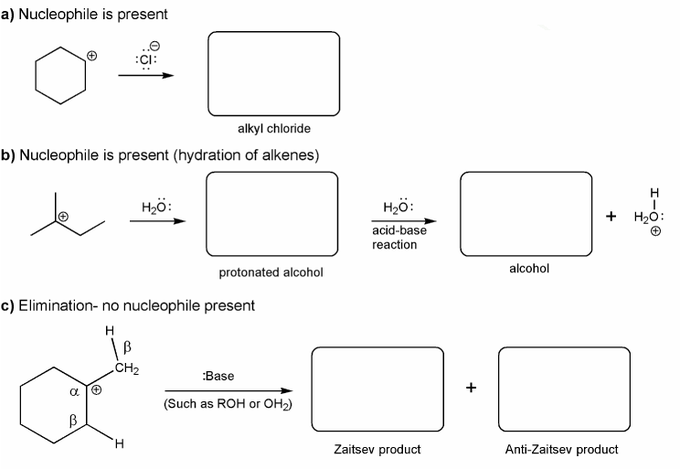
Carbocations aren't very stable and so don't last very long after they are formed.
Use curved arrows to show:
a) how a carbocation reacts with a halide ions to form an alkyl halide.
b) how a carbocation reacts with water to form an alcohol.
c) how a carbocation reacts with a base to form an alkene.
-
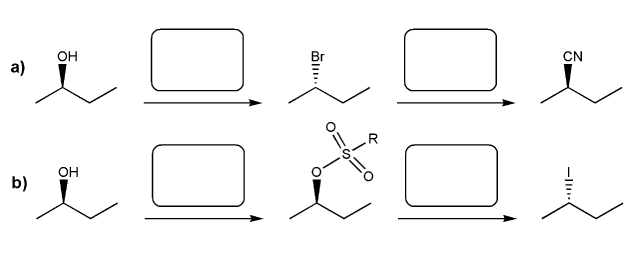
Problem # 537
Indicate the reagents necessary to carry out each transformation.
-
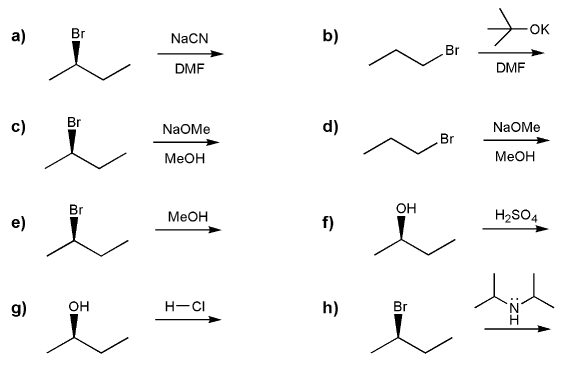
Problem # 560
For each reaction below, determine whether the primary reaction is SN1, SN2, E1, or E2, and then draw the product.
Note: Me = methyl (CH3)
-
Problem # 703
Show two ways to prepare the ether below from a combination of an alcohol and an alkyl halide via the Williamson ether synthesis.
Is one way better than the other? Why?

-
Problem # 337
Predict the product(s) of the reaction below, and used curved arrows to show a mechanism.
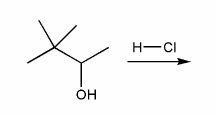
-
Problem # 744
After a sample of optically pure (S)-2-ethyl-cyclohexanone is dissolved in an aqueous solution for several hours, a significant loss of optical activity is observed. Explain.
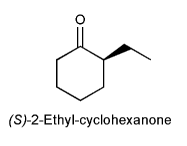
-
Problem # 531E2 elimination reactions require anti-coplanar geometry. (note: some textbooks call this anti-periplanar).Let's work through an E2 reaction, and rotate the molecule eblow into an anti-coplanar geometry to predict the product of this E2 reaction.
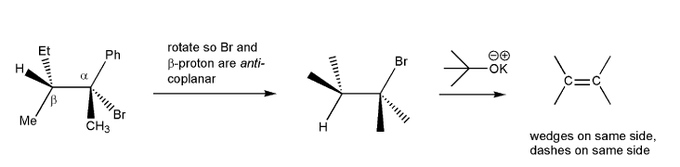
-
Problem # 530Let's work through anti and syn additions to alkenes.Show the product for each reaction below, and indicate whether the product will be a racemic mixture of enantiomers, or a meso compound (which is achiral).
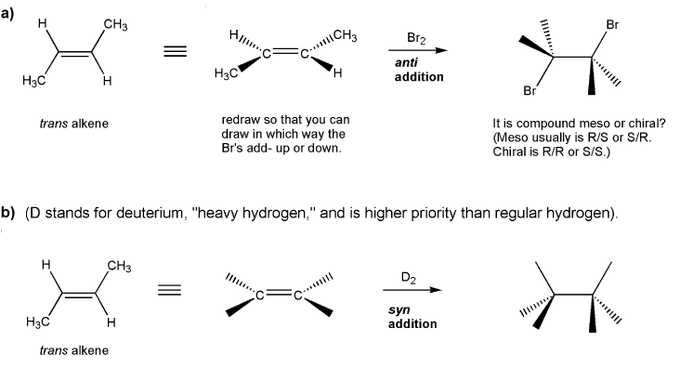
-
Problem # 529
Indicate the major organic product of the reaction below. Include stereochemistry.
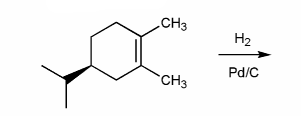
-
Problem # 528
Indicate which of the molecules below are chiral (if any).
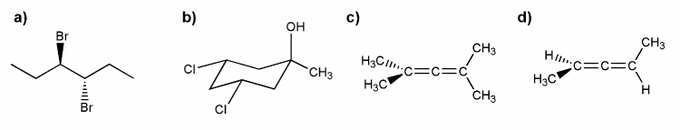
-
Problem # 1336
Determine a synthesis to prepare 2-chloro-4-methylpentane from 1-iodo-4-methylpentane.
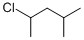 from
from 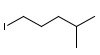
-
Problem # 343
For the reaction below, draw the structures of the chloronium ion intermediate and the final product.
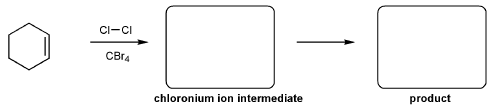
-
Problem # 345
For the reaction below, draw the structures of the radical intermediate and the final product.
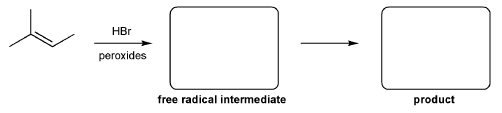
-
Problem # 520
Let's work through an alkene addition reaction. Draw the structures for each of the species in the three boxes below (3º carbocation, protonated thiol, and thiol). Also draw curved arrows to show electron movement. Note: thiol = RSH, like an alcohol, but with sulfur instead of oxygen.
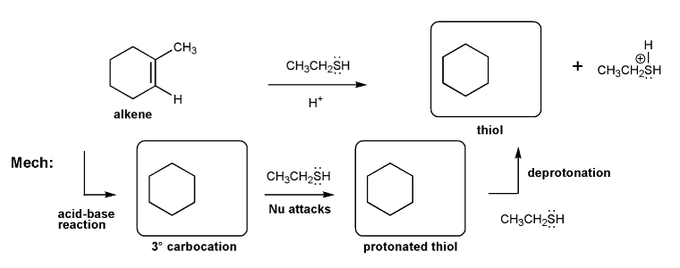
-
Problem # 562
Fill in the product for each reaction below. Indicate stereochemistry where appropriate.
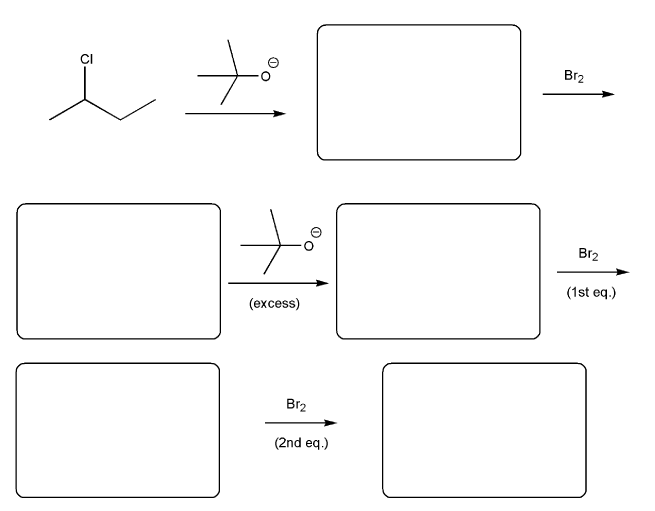
-
Problem # 561
Fill in the product for each reaction below. Indicate stereochemistry where appropriate.
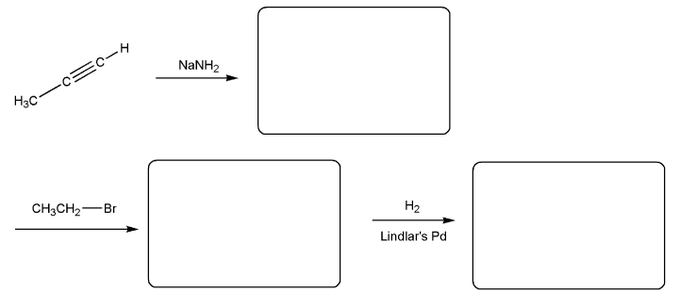
-
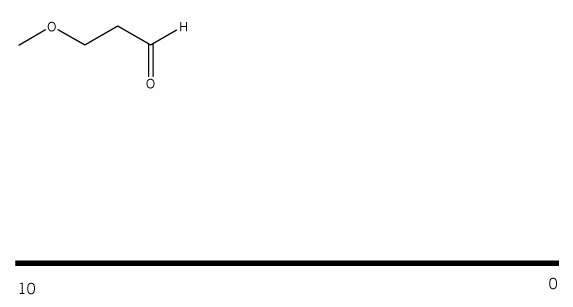
Problem # 679
Compound A (C5H12O) is oxidized using aqueous chromium (Jones reagent) to compound B (C5H10O2), which is then treated with methanol under acidic conditions to yield compound C (C6H12O2) and water.
The 1H NMR of compound C is shown below. Determine the structures of compounds A, B, and C.
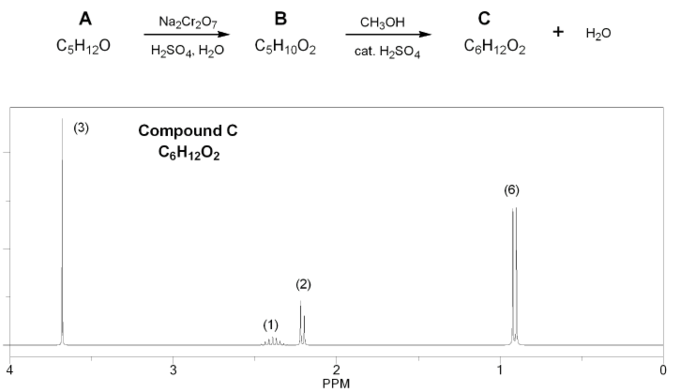
-
Problem # 673
Show how each compound can be prepared from an alkene containing 3 carbons (or less).
Each answer will involve the reaction of a Grignard with either a carbonyl or epoxide.
Note: epoxides are prepared from alkenes using a peroxy acid (epoxidation) such as mCPBA.
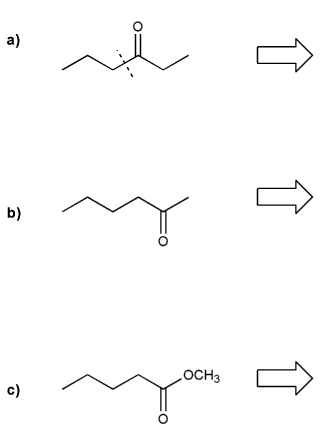
-
Problem # 749
Show how to prepare each compound below from propanal. I've marked the "cuts" for you.
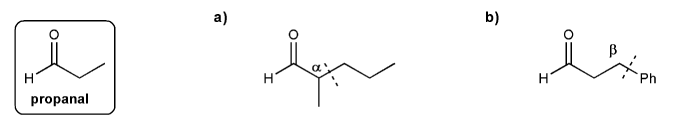
-
Problem # 714
Complete each synthesis below. All carbon sources must come from alkenes.
Each synthesis will involve protecting groups.
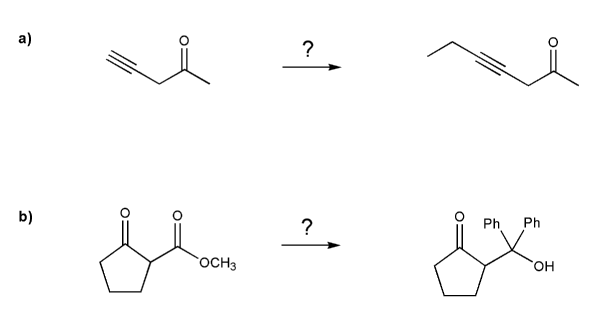
-
Problem # 702
Show how to prepare each compound starting from propylene oxide.

(Propylene oxide image below courtesy of Wikipedia.)
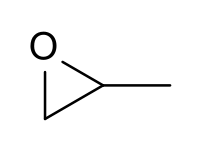
-
Problem # 699
Show how each compound can be prepared from the indicated starting material.
All carbon sources must contain three carbons or less.
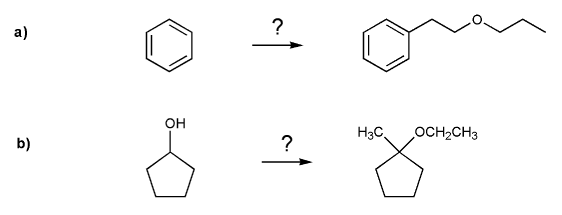
-
Problem # 668
Show how each alcohol can be prepared from a combination of a carbonyl and a Grignard reagent.
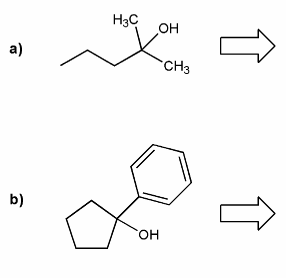
-
Problem # 700
Write out a mechanism for the reaction below using curved arrows. Be sure to include formal charges.
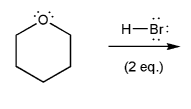
-
Problem # 677
Show a mechanism for the acid-catalyzed cyclization (condensation) of 1,4-butanediol.
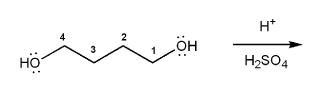
-
Problem # 670
Draw out the mechanism for the addition of excess phenyl Grignard to the carbonyl compound below.
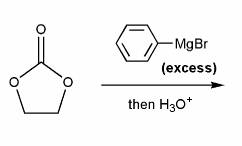
-
Problem # 674
Show a mechanism for the reduction of butyrolactone using LiAlH4.
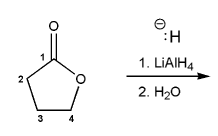
-
Problem # 672
Compound A has molecular formula C6H12O and shows a sharp peak at 1,710 cm-1 in its IR spectrum.
Treatment with 1 equivalent of phenyl Grignard yields compound B, which has formula C12H18O and whose IR shows a broad peak at 3,350 cm-1.
Compound B's 1H NMR spectrum is shown below. Determine the structures of compounds A and B.
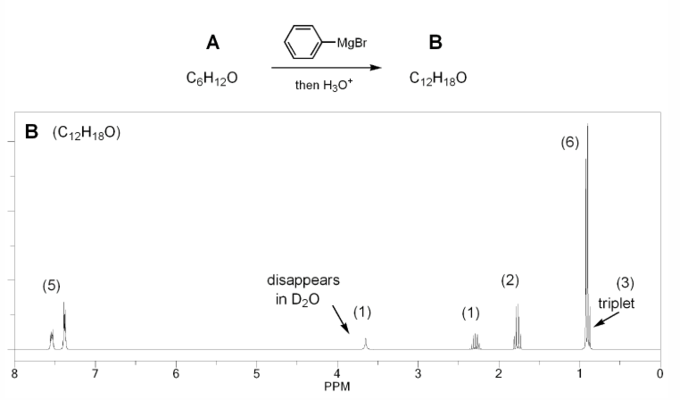
-
Problem # 659
The mass spec of 4-nonanone shows peaks at m/z = 58, 71, 86, 99.
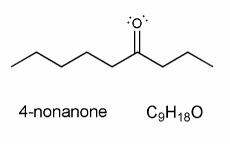
Using curved arrows or hooks, show how each of these fragments can form via alpha cleavage or the McLafferty rearrangement. (and draw the structure of the indicated species in the appropriate box).
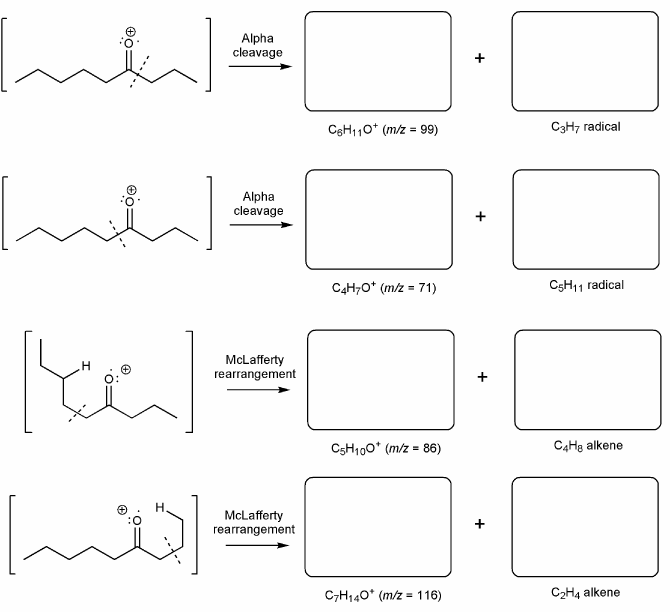
-
Problem # 658
The mass spec of methyl ethyl ether shows peaks at m/z = 45 and 59.
Use hooks to show the alpha cleavages that result in these two fragments.

-
Problem # 730
N,N-dimethylformamide (DMF) is shown below. Based on its structure, you might expect to see only one -CH3 signal in the 1H NMR spectrum. But instead DMF shows two different -CH3 signals. Explain.
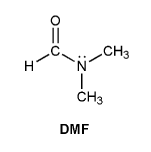
-
Problem # 666
The 1H and 13C NMR spectra of an unknown compound are shown below. The compound's mass spectrum shows a molecular ion with m/z ratio of 122. Determine the structure of this compound.
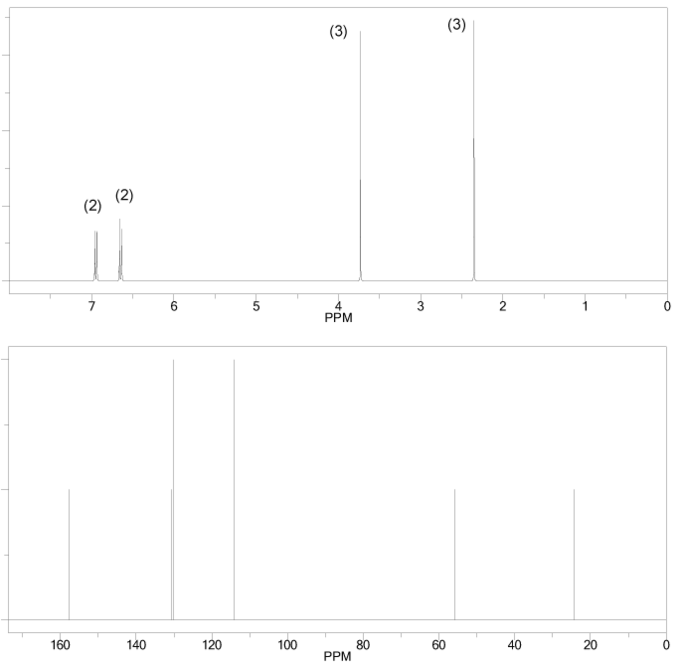
-
Problem # 665
The 1H and 13C NMR spectra of an unknown compound are shown below. The compound's mass spectrum shows a molecular ion with m/z ratio of 86. Determine the structure of this compound.
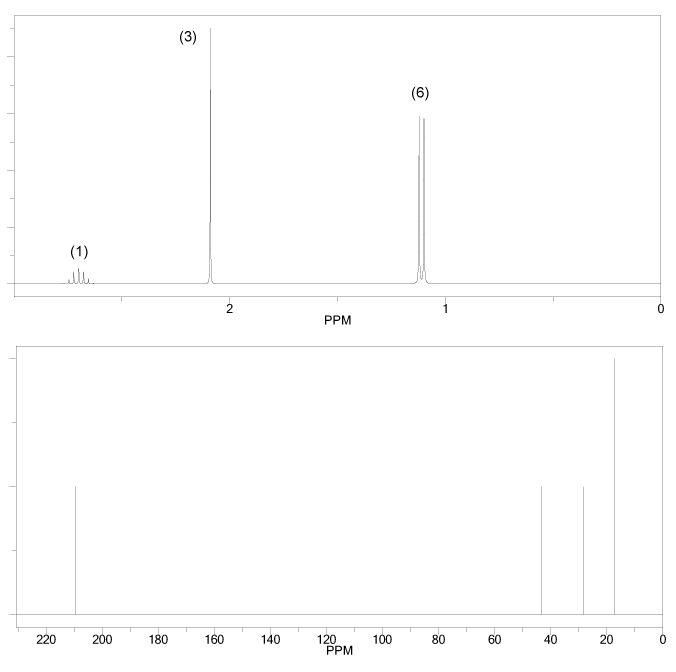
-
Problem # 663
The 1H and 13C NMR spectra of a compound with chemical formula C4H6O2 are shown below. The compound's IR spectrum shows a sharp peak at 1,700 cm-1. Determine the structure of this compound.
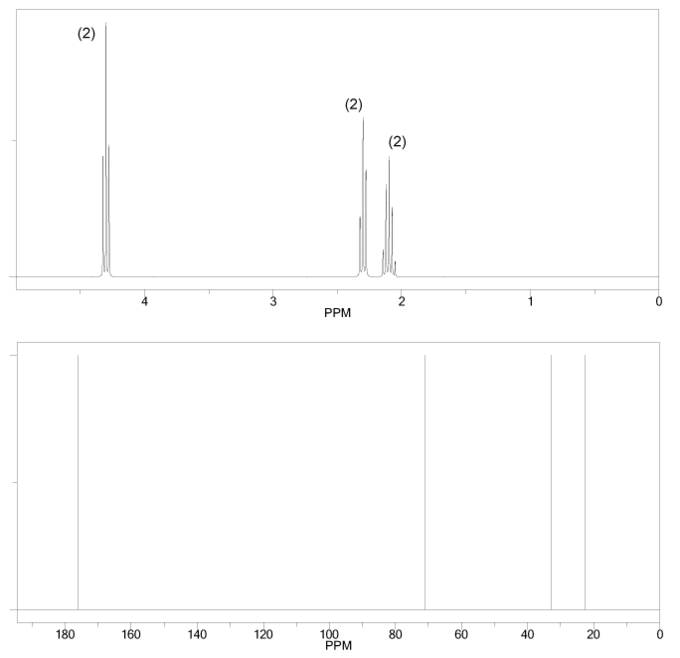
-
Problem # 662
The 1H and 13C NMR spectra of a compound with chemical formula C10H14O are shown below. The compound's IR spectrum shows a broad peak at 3,300 cm-1. Determine the structure of this compound.
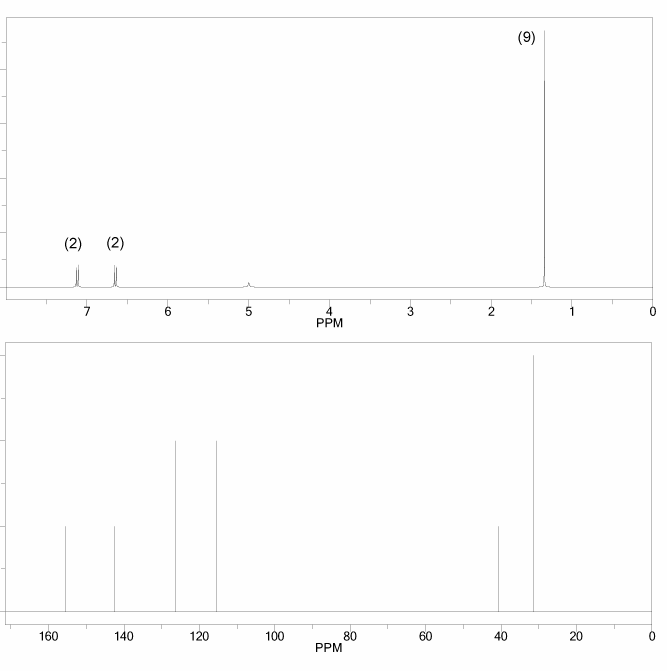
-
Problem # 660
Using your knowledge of 1H NMR, predict the NMR spectrum for the compound below. (draw out the spectrum you would expect to see). Be sure to include:
- peak integrations
- peak multiplicities
- chemical shifts (approximate)