MS 1324 - Grignard
Solutions can be seen at mendelset.com/ms/1324
Description: Introductory Grignard problems
Total Problems: 4
-
Problem # 671
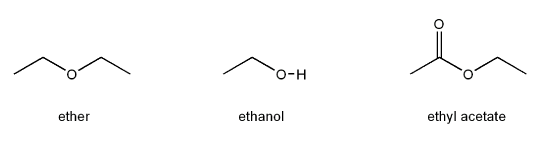
You may have noticed that the "solvent of choice" for many organometallic compounds such as Grignard reagents is ether (short for diethyl ether).
Why is it that for Grignard reactions this solvent is used over ethyl acetate, or protic solvents such as ethanol?
-
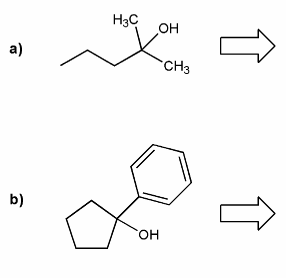
Problem # 668
Show how each alcohol can be prepared from a combination of a carbonyl and a Grignard reagent.
-
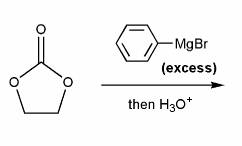
Problem # 670
Draw out the mechanism for the addition of excess phenyl Grignard to the carbonyl compound below.
-
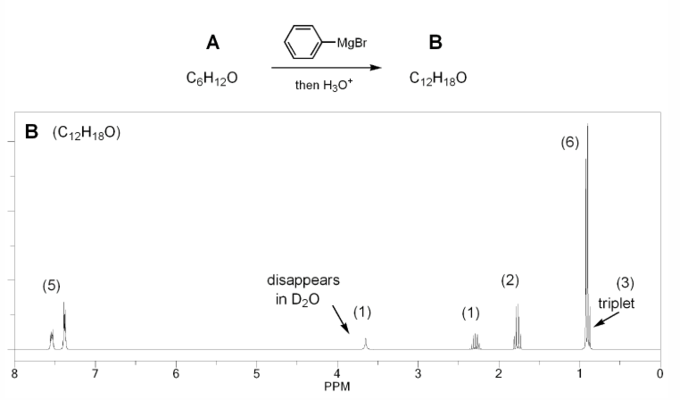
Problem # 672
Compound A has molecular formula C6H12O and shows a sharp peak at 1,710 cm-1 in its IR spectrum.
Treatment with 1 equivalent of phenyl Grignard yields compound B, which has formula C12H18O and whose IR shows a broad peak at 3,350 cm-1.
Compound B's 1H NMR spectrum is shown below. Determine the structures of compounds A and B.