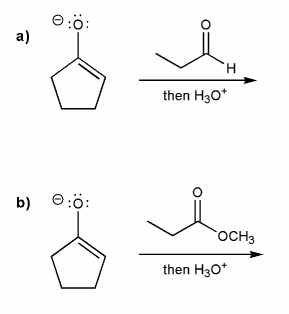
Enolates are nucleophiles and react with a variety of electrophiles.
Carbonyls are electrophiles. But aldehydes/ketones and esters/acid chlorides often form different products.
Use curved arrows to draw a mechanism for each reaction below. How do the two products differ?
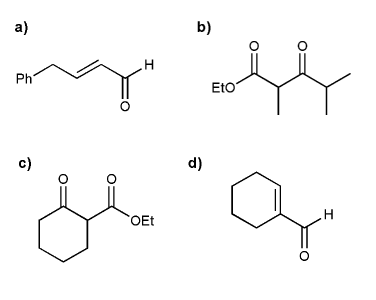
Show what combination of aldehyde, ketone, and/or ester can prepare each compound below. Every compound is a Claisen or aldol product.
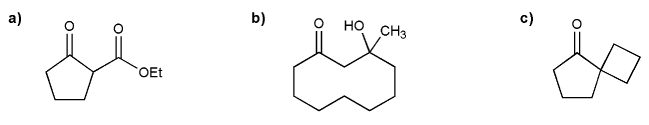
Show a combination of enolate (nucleophile) and electrophile that can produce each compound below.
Remember that all enolates come from carbonyls.
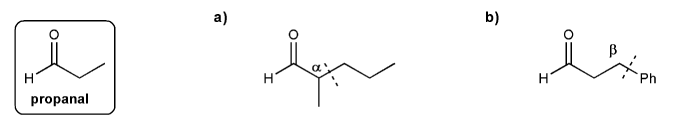
Show how to prepare each compound below from propanal. I've marked the "cuts" for you.
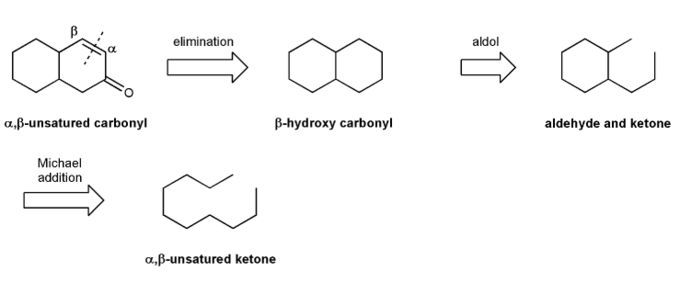
Let's work through a Robinson annulation.
Work backwords to determine the starting materials needed to produce each intermediate below, then show a mechanism for the overall reaction.