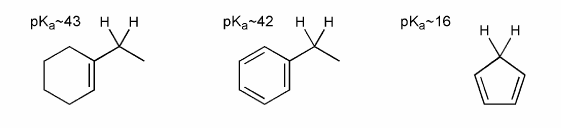
Rationalize the follwing pKa values. Explain your answer in terms of the stabilites of the conjugates bases of each acid.
Note: the lower the pKa, the stronger the acid.
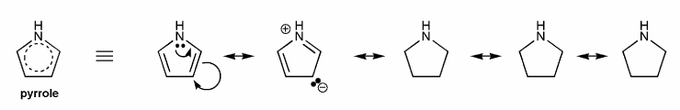
Pyrrole is an example of a heteroaromatic compound: it contains a heteroatom (atom that is not carbon or hydrogen, such as N, O, S, etc.), and is aromatic.
Because pyrrole is aromatic, we should be able to draw many resonance forms- usually as many resonance forms as sides (in this case, five sides, so five resonane forms).
Draw all resonance forms for pyrrole. (I've started you off.)
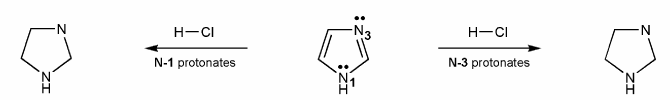
Imidazole (shown below) has two nitrogen atoms, N-1 and N-3. Which nitrogen is more basic?
To answer this problem, draw the product after each nitrogen protonates, and compare their stabilities. Explain your reasoning.