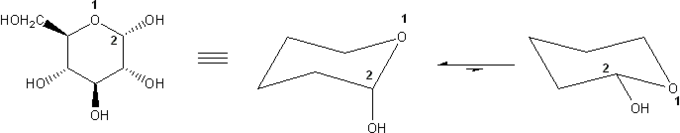
α-D-Glucose is shown below. Draw its two chair forms. Which conformation is more stable? Explain.
I recommend using the common convention wedge = "up" and dash = "down."
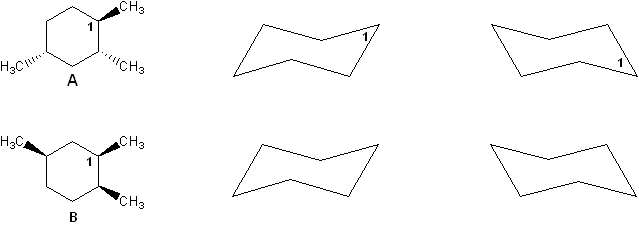
Two stereoisomers of trimethylcyclohexane are shown below (compounds A and B). Compare cyclohexane chair forms to determine which isomer has a lower heat of combustion. Explain your reasoning.
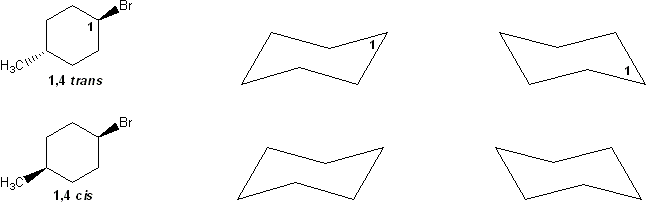
For a molecule to undergo an E2 reaction, the leaving group and the beta-proton must be in an anti-coplanar conformation (one atom straight up, the other straight down). Based on this, which compound undergoes E2 reaction with KOtBu faster? Why?