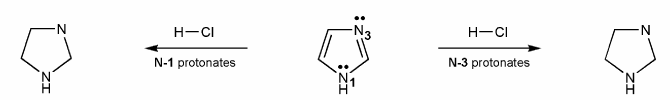
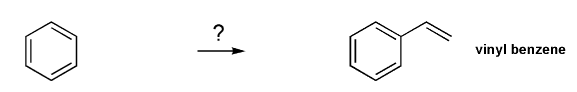Imidazole (shown below) has two nitrogen atoms, N-1 and N-3. Which nitrogen is more basic?
To answer this problem, draw the product after each nitrogen protonates, and compare their stabilities. Explain your reasoning.
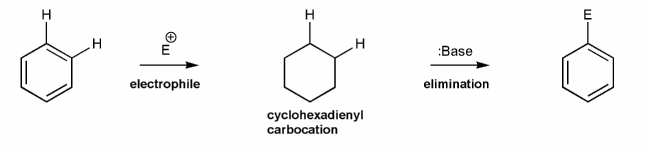
Use curved arrows to draw a mechanism for the generic electrophilic aromatic substitution (EAS) reaction below.
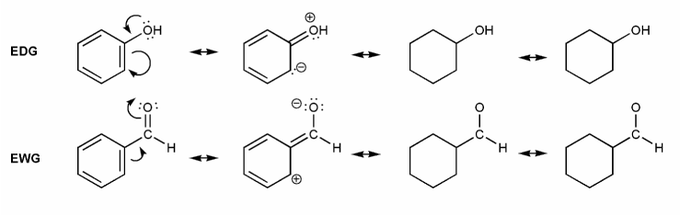
Let's draw resonance forms to see why some groups are EDG or EWG. (I've started you off)
Where are the positive or negative charges placed in EDG/EWG? (ortho/meta/para) Why would this affect EAS reactions?
Note: EDG = electron donating group, EWG = electron withdrawing group
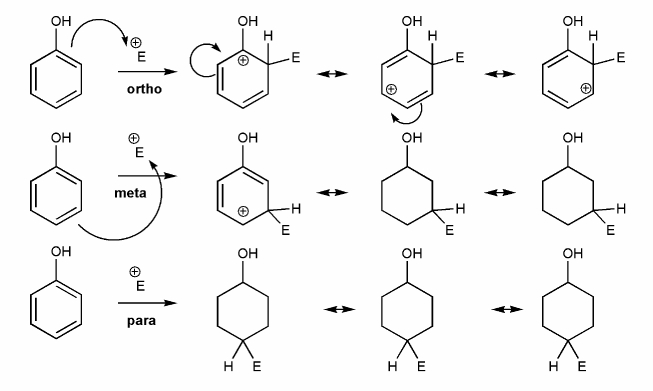
-OR is an EDG and an ortho-para director. Let's draw an EAS reaction's cyclohexadienyl cation intermediates to demonstrate why this is true. I've started you off.
What's good about ortho/para? What's bad about meta?
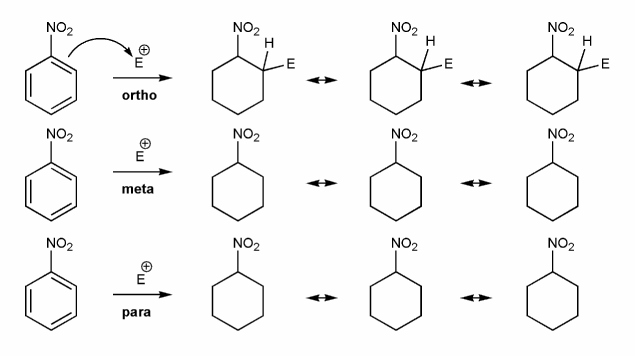
-NO2 is an EWG and a meta director. Let's draw an EAS reaction's cyclohexadienyl cation intermediates to demonstrate why this is true. I've started you off.
What's good about meta? What's bad about ortho/para?
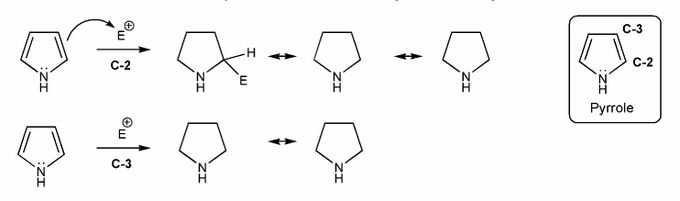
Pyrrole undergoes eletrophilic aromatic substitution at C-2. Let's compare the resonance forms of EAS carbocation intermediates to see why this is the case. What do you think? Why C-2 and not C-3?
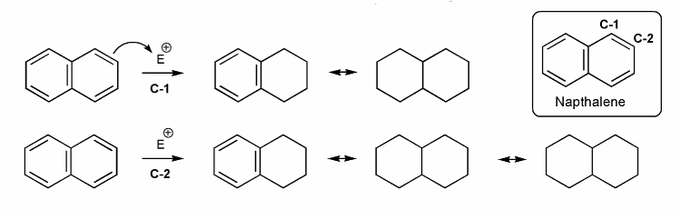
Naphthalene undergoes eletrophilic substitution at C-1.
Why is this the case, even though substitution at C-2 gives more resonance forms?
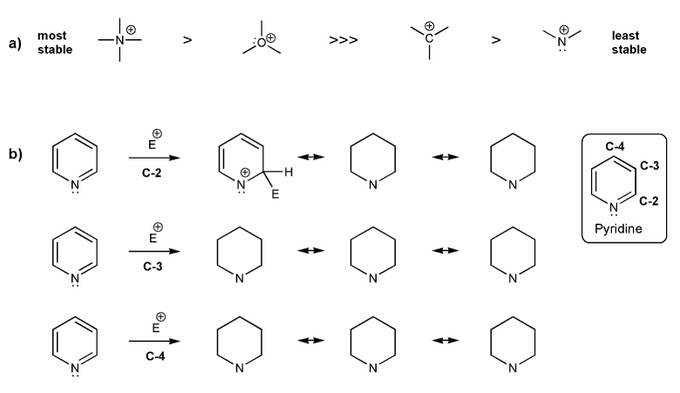
a) Rationalize the relative stabilities of the cation species below.
b) Pyridine undergoes eletrophilic substitution at C-3. Let's compare the resonance forms of EAS carbocation intermediates to see why this is the case. Consider part a) in your explanation.
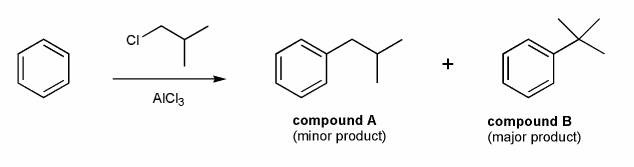
A chemist tried to prepare compound A from benzene via Friedel-Crafts alkylation and instead produced compound B.
Why did this happen? How could the chemist prepare compound A?
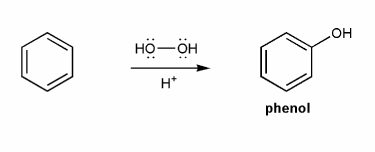
Phenol can be prepared from benzene and hydrogen peroxide in the presence of a really strong acid. Propose a mechanism for this reaction.
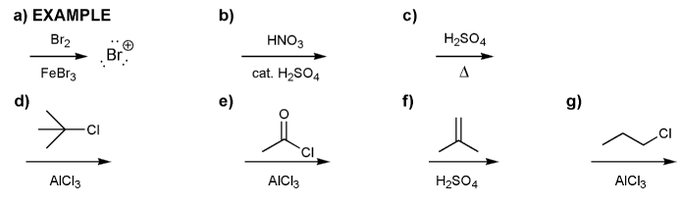
Indicate the eletrophile formed by each set of reagents/conditions below.
Draw in the arrows to show the electron flow and resonance forms in the nucleophilic aromatic substitution reaction below.
Note: Depending on the textbook, nucleophilic aromatic substitution is referred to as NAS, SNAr, or addition-elimination.
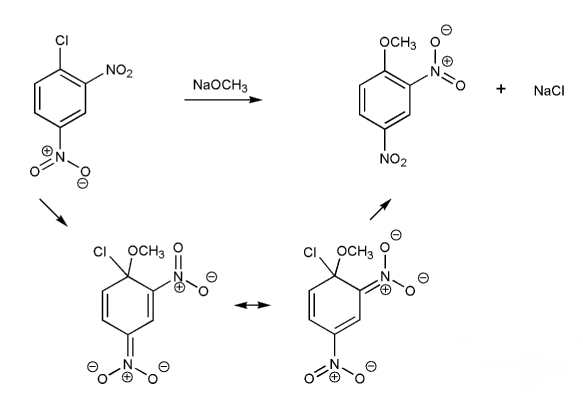
Draw a mechanism for the nucleophilic aromatic substitution (SNAr) reaction below. Show all resonance forms of the intermediate.
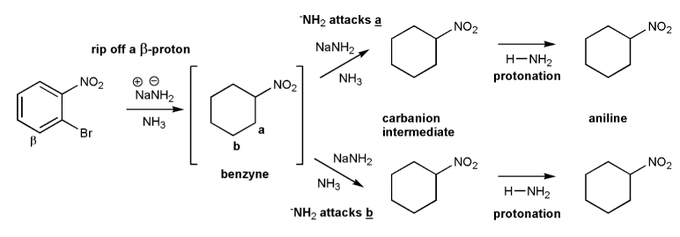
Let's go through a benzyne reaction (also called elimination-addition).
In the reaction below, the strong base (NaNH2) will form a benzyne intermediate, which when forms either ortho nitroaniline or meta nitroaniline.
Used curved arrows to show the formation of each intermediate and the final products.
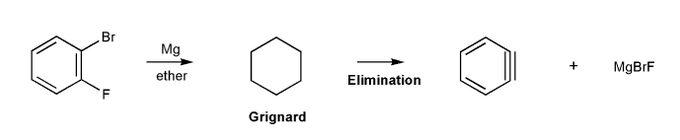
Let's go through another way to make benzyne.
First, let's form a Grignard reagent. Then, let's elminate to form benzyne.
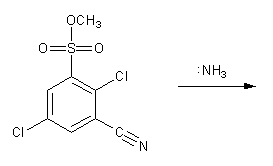
Draw the structure of the major organic product from each reaction sequence.
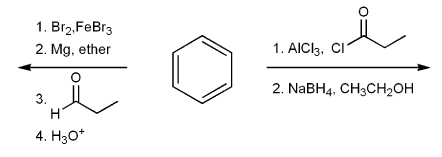
Show how to prepare vinyl benzene from benzene.