Rank each group of acids in order of decreasing acidity. (1 = most acidic)
Explain your reasoning. You will have to use more than one rule in your explanation (resonance, electronegativity, atomic radius, etc.).
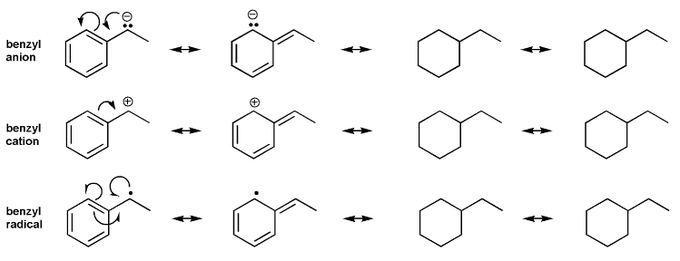
Draw all resonance forms for each species.
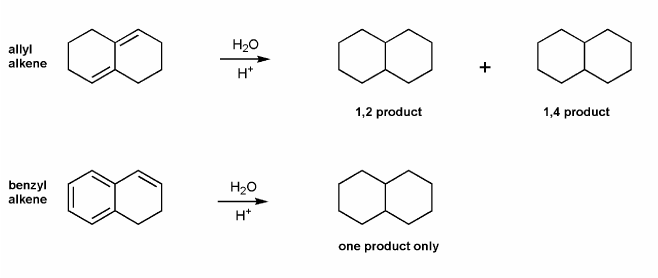
Draw all products for the two reactions below.
The allylic alkene gives two products- the 1,2 product, and the 1,4 product.
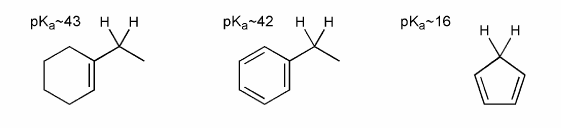
Rationalize the follwing pKa values. Explain your answer in terms of the stabilites of the conjugates bases of each acid.
Note: the lower the pKa, the stronger the acid.
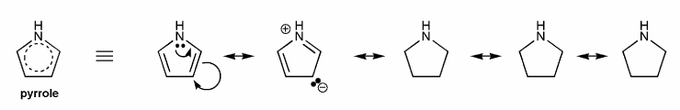
Pyrrole is an example of a heteroaromatic compound: it contains a heteroatom (atom that is not carbon or hydrogen, such as N, O, S, etc.), and is aromatic.
Because pyrrole is aromatic, we should be able to draw many resonance forms- usually as many resonance forms as sides (in this case, five sides, so five resonane forms).
Draw all resonance forms for pyrrole. (I've started you off.)
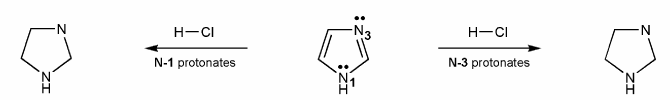
Imidazole (shown below) has two nitrogen atoms, N-1 and N-3. Which nitrogen is more basic?
To answer this problem, draw the product after each nitrogen protonates, and compare their stabilities. Explain your reasoning.
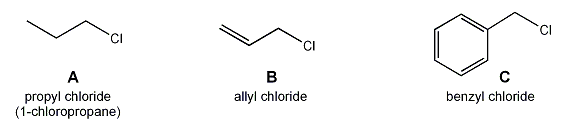
Allylic and benzylic halides tend to undergo both SN1 and SN2 substitution reactions at a faster rate than their alkyl counterparts.
For example, both allyl chloride and benzyl chloride undergo SN2 reaction at a faster rate than propyl chloride.
The same holds true for SN1 reactions: a 2° allyl or benzyl halide undergoes SN1 reaction faster than a 2° alkyl halide. Explain.