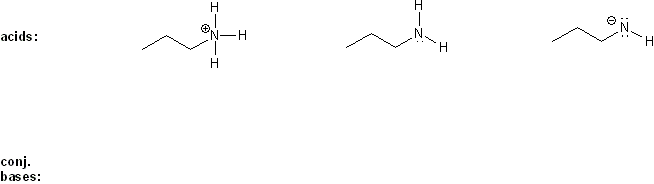
Draw the conjugate base forms of each acid listed below, then rank the acids in order or decreasing acidity (1 = most acidic).
Explain your reasoning.
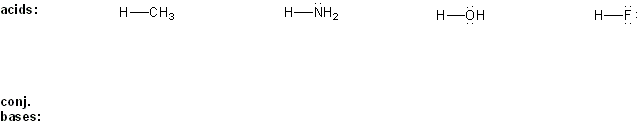
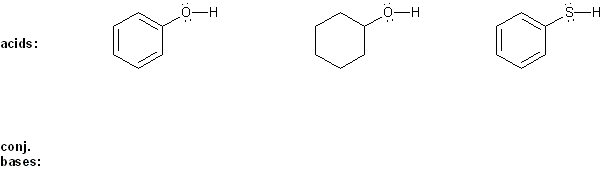
Draw the conjugate base form of each acid listed below, then rank the acids in order or decreasing acidity (1 = most acidic).
Explain your reasoning.
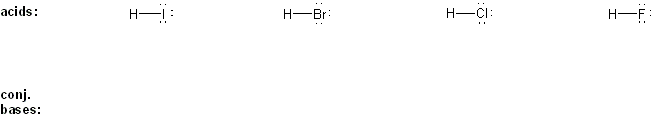
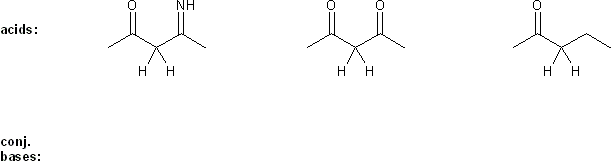
Draw the conjugate base form of each acid listed below, then rank the acids in order or decreasing acidity (1 = most acidic).
Explain your reasoning.
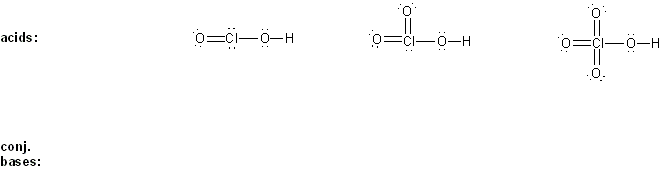
Draw the conjugate base form of each acid listed below, then rank the acids in order or decreasing acidity (1 = most acidic).
Explain your reasoning.
Rank each group of acids in order of decreasing acidity. (1 = most acidic)
Explain your reasoning. You will have to use more than one rule in your explanation (resonance, electronegativity, atomic radius, etc.).
Rank each group of acids in order of decreasing acidity. (1 = most acidic)
Explain your reasoning. You will have to use more than one rule in your explanation (resonance, electronegativity, atomic radius, etc.).
Rank the group of molecules below in in order of decreasing basicity. (1 = most basic)
Explain your reasoning.
![]()
Rank the group of molecules below in in order of decreasing basicity. (1 = most basic)
Explain your reasoning.
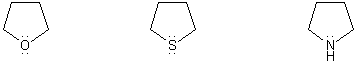
Rank the following anions in order of decreasing stability (1 = most stable)
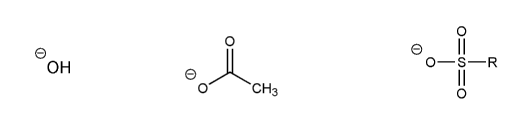
Rank the following anions in order of decreasing stability (1 = most stable)

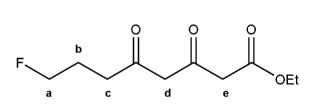
The molecule below has five different types of hydrogens (A through E). Rank each in order of decreasing acidity.
(1 = most acidic). Explain your reasoning.