

Convert each formula to a carbon skeleton diagram, or vice-versa.
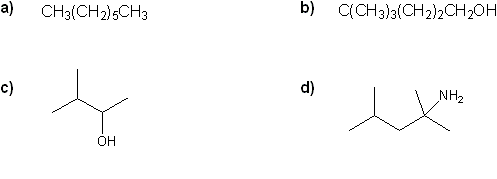
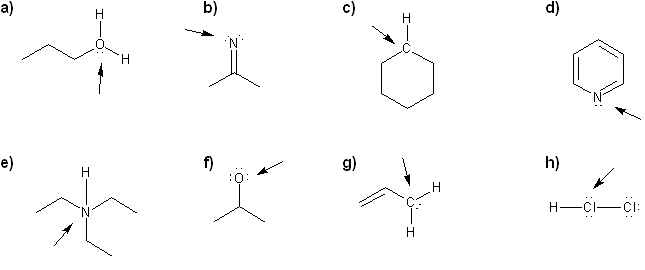
For each molecule, determine the formal charge of the indicated atom.
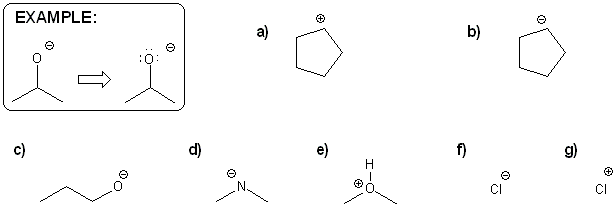
For each molecule below, draw in all implied lone pairs and/or protons (hydrogens) based on the formal charge shown.
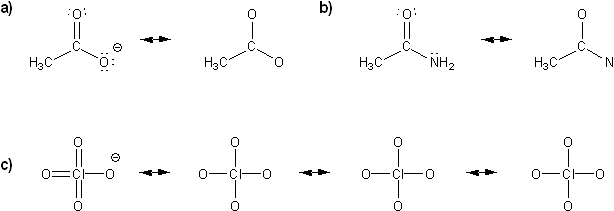
Draw all possible resonance forms for each structure below. Use curved arrows.
Note that some structures only show charge, and not implied protons or lone pairs!
Draw all possible resonance forms for each structure below. Use curved arrows.
Note that some structures only show charge, and not implied protons or lone pairs!
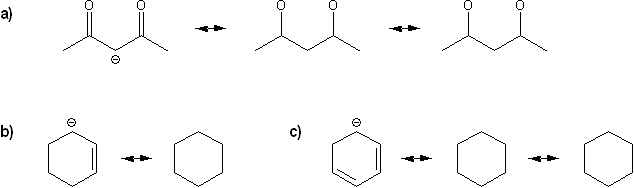
Draw all possible resonance forms for each structure below. Use curved arrows.
Note that some structures only show charge, and not implied protons or lone pairs!
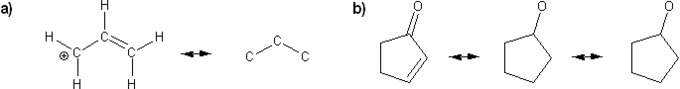
Draw all possible resonance forms for each structure below. Use curved arrows.
Note that some structures only show charge, and not implied protons or lone pairs!
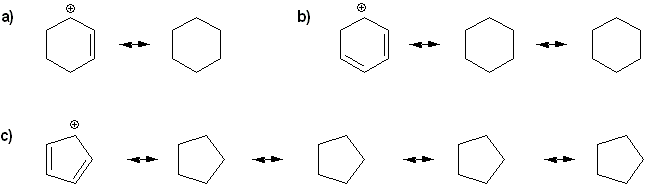
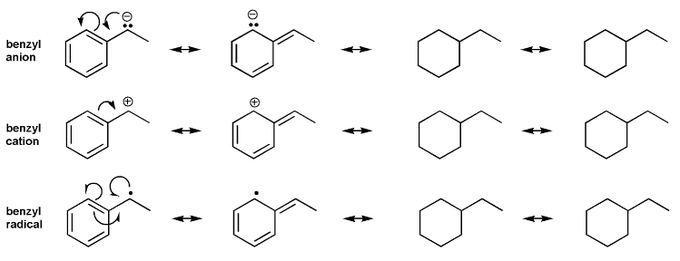
Draw all resonance forms for each species.
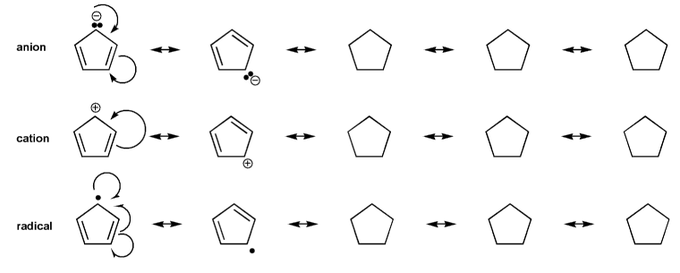
Draw all resonance forms for each species.
Rank the following compounds in order of decreasing boiling point.
Also, make a guess about their relative solubilities in water. Explain your reasoning.
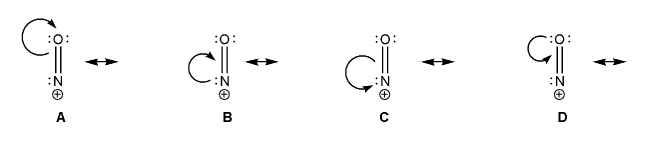
The nitrosyl cation is shown below. Also shown are several proposed resonance arrows, only one of which is correct.
Draw the resonance forms that would follow from each set of arrows, and include formal charges. Which one is the correct resonance form? Explain your reasoning.
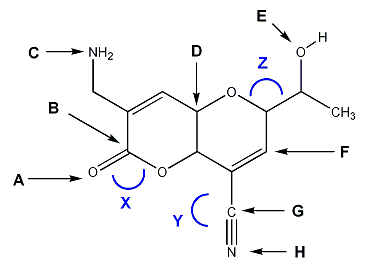
For the molecule shown below, indicate the hybridization (sp3, sp2, sp, etc.) of atoms A through H, and the bond angles of X, Y, and Z.
Rank each set of compounds in order of decreasing boiling point (1 = highest boiling point):
a) ethane, n-octane, n-pentane
b) n-butane, 1-butanol, 1-chlorobutane.
c) n-octane, 2-methylheptane, 2,5-dimethylhexane
(Note that the n- prefix before an alkane just means that it's one chain, without any branching.)