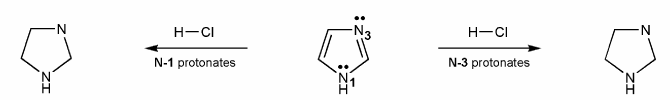
Imidazole (shown below) has two nitrogen atoms, N-1 and N-3. Which nitrogen is more basic?
To answer this problem, draw the product after each nitrogen protonates, and compare their stabilities. Explain your reasoning.
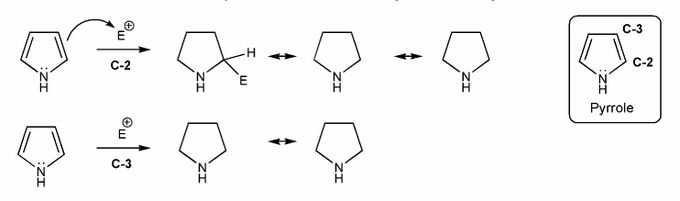
Pyrrole undergoes eletrophilic aromatic substitution at C-2. Let's compare the resonance forms of EAS carbocation intermediates to see why this is the case. What do you think? Why C-2 and not C-3?
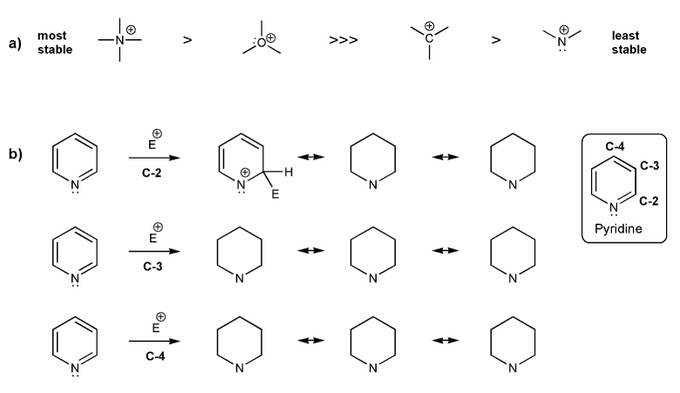
a) Rationalize the relative stabilities of the cation species below.
b) Pyridine undergoes eletrophilic substitution at C-3. Let's compare the resonance forms of EAS carbocation intermediates to see why this is the case. Consider part a) in your explanation.