Rank each group of acids in order of decreasing acidity. (1 = most acidic)
Explain your reasoning. You will have to use more than one rule in your explanation (resonance, electronegativity, atomic radius, etc.).
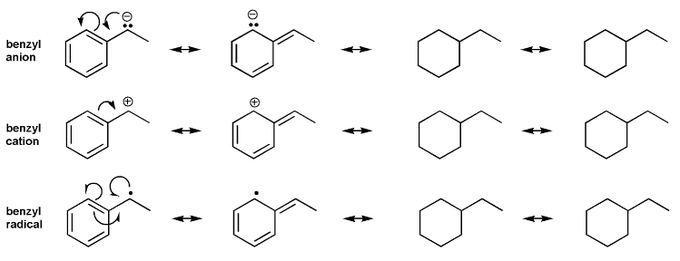
Draw all resonance forms for each species.
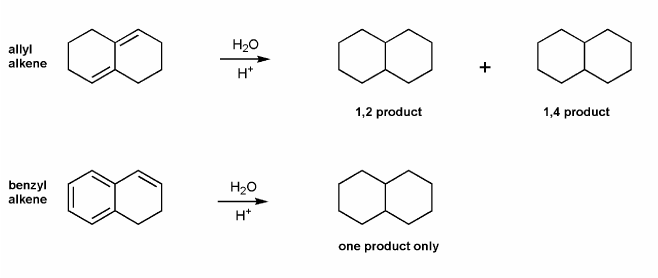
Draw all products for the two reactions below.
The allylic alkene gives two products- the 1,2 product, and the 1,4 product.
Draw in the arrows to show the electron flow and resonance forms in the nucleophilic aromatic substitution reaction below.
Note: Depending on the textbook, nucleophilic aromatic substitution is referred to as NAS, SNAr, or addition-elimination.
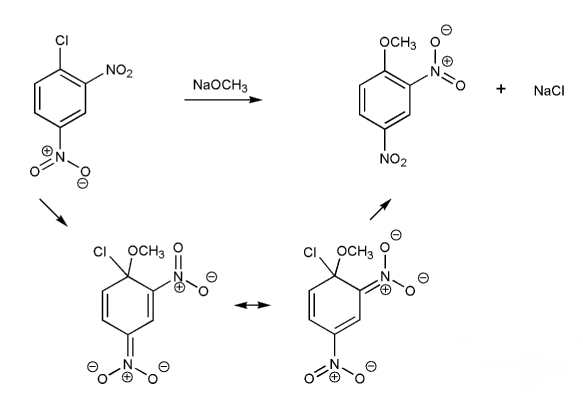
Draw a mechanism for the nucleophilic aromatic substitution (SNAr) reaction below. Show all resonance forms of the intermediate.
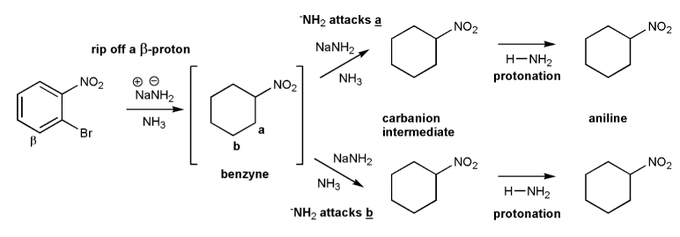
Let's go through a benzyne reaction (also called elimination-addition).
In the reaction below, the strong base (NaNH2) will form a benzyne intermediate, which when forms either ortho nitroaniline or meta nitroaniline.
Used curved arrows to show the formation of each intermediate and the final products.
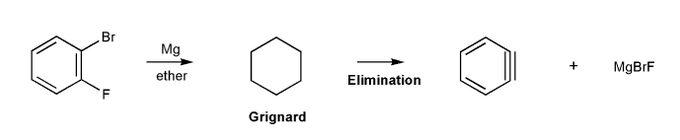
Let's go through another way to make benzyne.
First, let's form a Grignard reagent. Then, let's elminate to form benzyne.
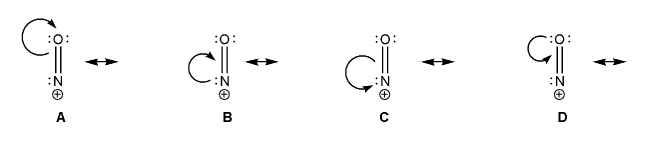
The nitrosyl cation is shown below. Also shown are several proposed resonance arrows, only one of which is correct.
Draw the resonance forms that would follow from each set of arrows, and include formal charges. Which one is the correct resonance form? Explain your reasoning.
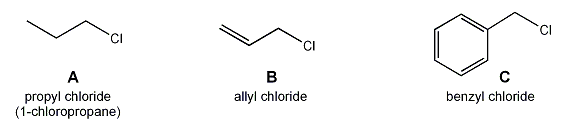
Allylic and benzylic halides tend to undergo both SN1 and SN2 substitution reactions at a faster rate than their alkyl counterparts.
For example, both allyl chloride and benzyl chloride undergo SN2 reaction at a faster rate than propyl chloride.
The same holds true for SN1 reactions: a 2° allyl or benzyl halide undergoes SN1 reaction faster than a 2° alkyl halide. Explain.