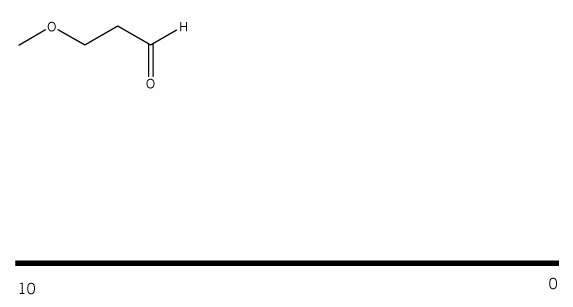
Using your knowledge of 1H NMR, predict the NMR spectrum for the compound below. (draw out the spectrum you would expect to see). Be sure to include:
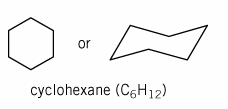
The proton NMR of cyclohexane gives only one peak when the NMR is run at room temperature.
But when the temperature is lowered to -100 ºC the proton NMR spectrum shows two peaks. Explain.
The 1H and 13C NMR spectra of a compound with chemical formula C10H14O are shown below. The compound's IR spectrum shows a broad peak at 3,300 cm-1. Determine the structure of this compound.
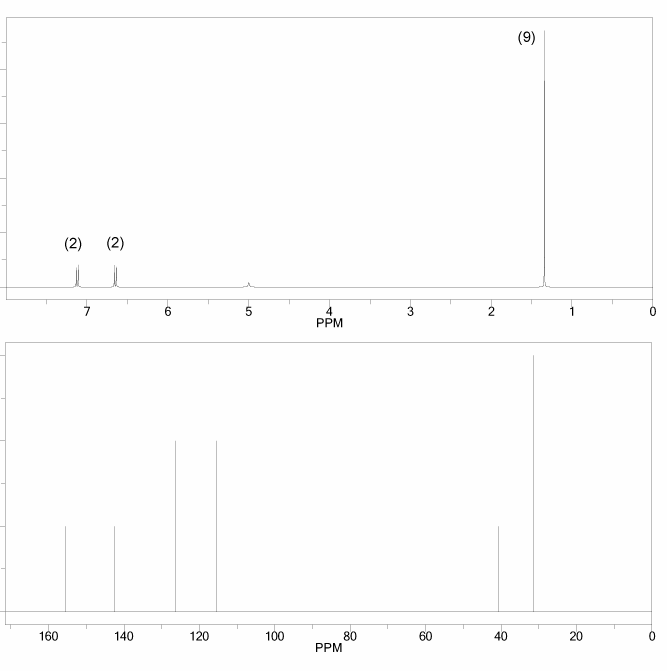
The 1H and 13C NMR spectra of a compound with chemical formula C4H6O2 are shown below. The compound's IR spectrum shows a sharp peak at 1,700 cm-1. Determine the structure of this compound.
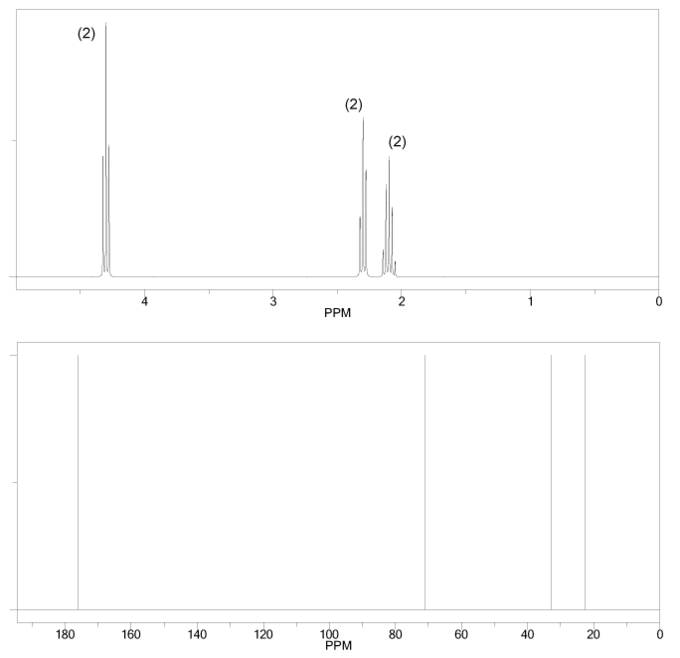
The 1H and 13C NMR spectra of an unknown compound are shown below. The compound's mass spectrum shows a molecular ion with m/z ratio of 86. Determine the structure of this compound.
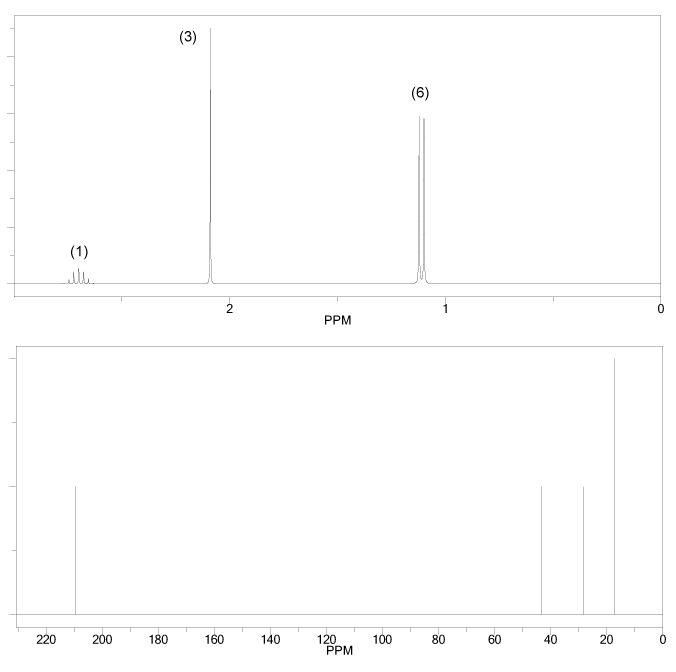
The 1H and 13C NMR spectra of an unknown compound are shown below. The compound's mass spectrum shows a molecular ion with m/z ratio of 122. Determine the structure of this compound.
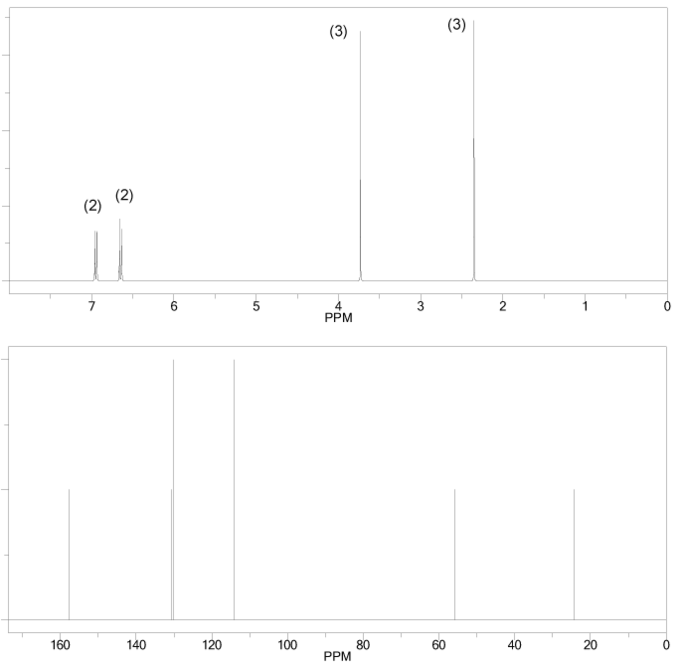
Compound A has molecular formula C6H12O and shows a sharp peak at 1,710 cm-1 in its IR spectrum.
Treatment with 1 equivalent of phenyl Grignard yields compound B, which has formula C12H18O and whose IR shows a broad peak at 3,350 cm-1.
Compound B's 1H NMR spectrum is shown below. Determine the structures of compounds A and B.
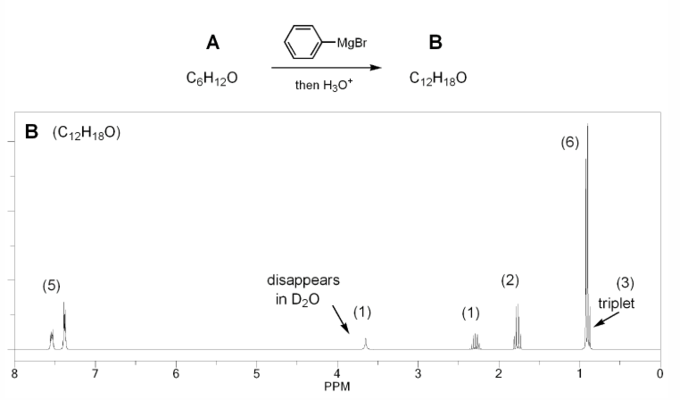
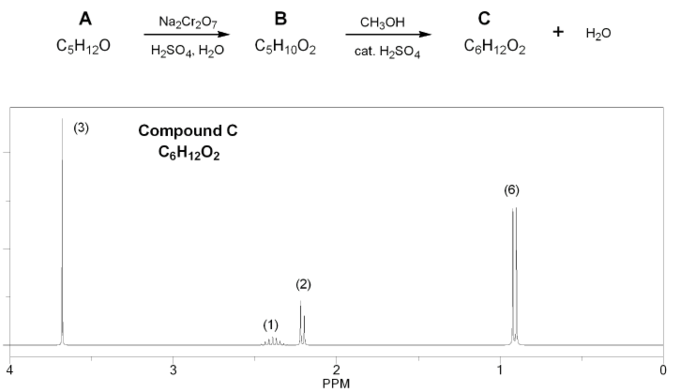
Compound A (C5H12O) is oxidized using aqueous chromium (Jones reagent) to compound B (C5H10O2), which is then treated with methanol under acidic conditions to yield compound C (C6H12O2) and water.
The 1H NMR of compound C is shown below. Determine the structures of compounds A, B, and C.
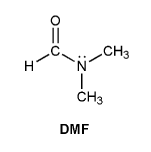
N,N-dimethylformamide (DMF) is shown below. Based on its structure, you might expect to see only one -CH3 signal in the 1H NMR spectrum. But instead DMF shows two different -CH3 signals. Explain.