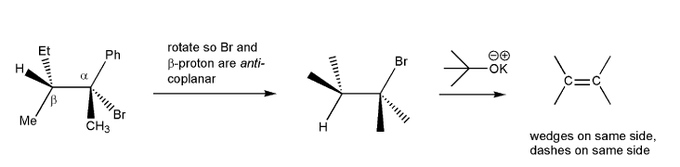
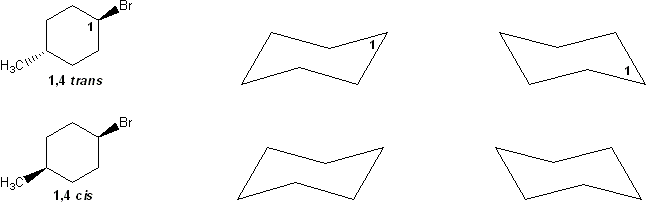
For a molecule to undergo an E2 reaction, the leaving group and the beta-proton must be in an anti-coplanar conformation (one atom straight up, the other straight down). Based on this, which compound undergoes E2 reaction with KOtBu faster? Why?
Carbocations aren't very stable and so don't last very long after they are formed.
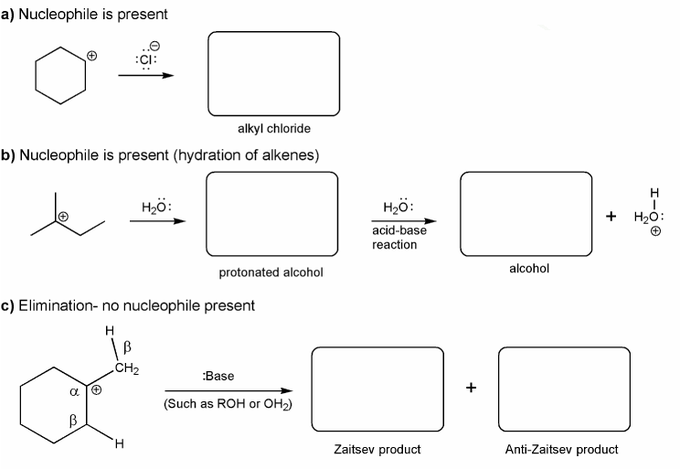
Use curved arrows to show:
a) how a carbocation reacts with a halide ions to form an alkyl halide.
b) how a carbocation reacts with water to form an alcohol.
c) how a carbocation reacts with a base to form an alkene.
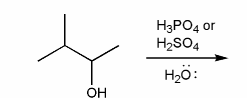
Predict the product(s) of the reaction below, and used curved arrows to show a mechanism.
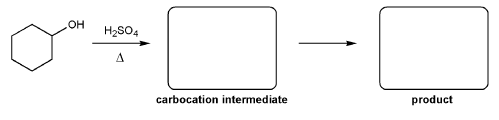
For the reaction below, draw the structures of the carbocation intermediate and the final product.
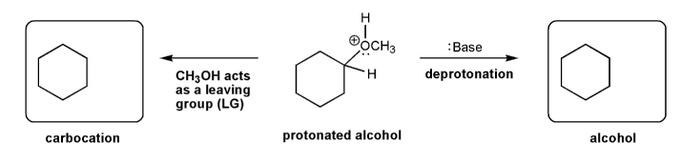
The alcohol below is protonated and contains an oxygen with a positive charge. Using curved arrows, show the two "legal moves" that result in a neutral oxygen.
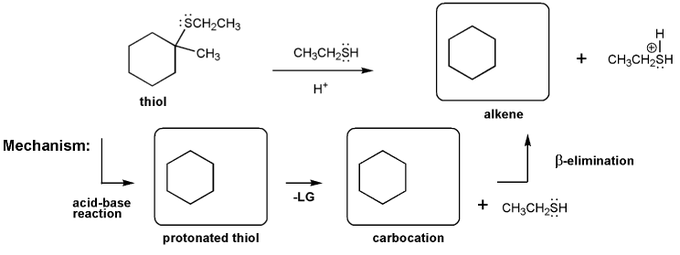
Let's work through an elimination reaction. Draw the structures for each of the species in the three boxes below (protonated thiol, carbocation, and alkene). Also draw curved arrows to show electron movement.