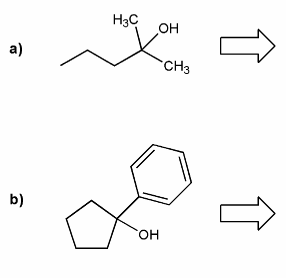
Show how each alcohol can be prepared from a combination of a carbonyl and a Grignard reagent.
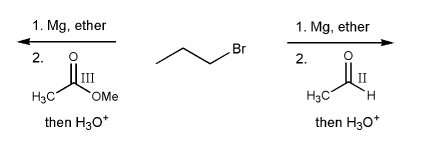
In your own words, what is the major difference in the addition of a Grignard reagent to an oxidation state III carbonyl (ester/acid chloride) versus an oxidation state II carbonyl? (aldehyde/ketone)
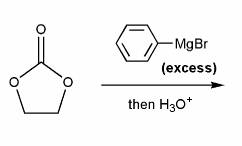
Draw out the mechanism for the addition of excess phenyl Grignard to the carbonyl compound below.
You may have noticed that the "solvent of choice" for many organometallic compounds such as Grignard reagents is ether (short for diethyl ether).
Why is it that for Grignard reactions this solvent is used over ethyl acetate, or protic solvents such as ethanol?
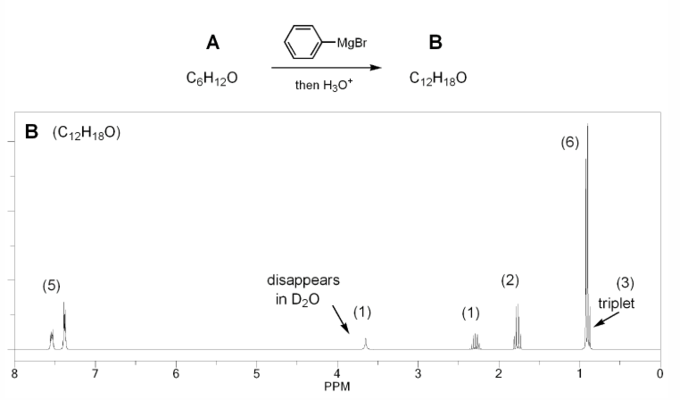
Compound A has molecular formula C6H12O and shows a sharp peak at 1,710 cm-1 in its IR spectrum.
Treatment with 1 equivalent of phenyl Grignard yields compound B, which has formula C12H18O and whose IR shows a broad peak at 3,350 cm-1.
Compound B's 1H NMR spectrum is shown below. Determine the structures of compounds A and B.
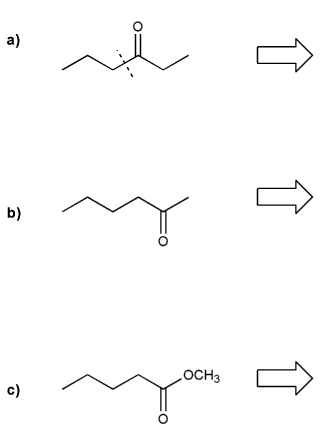
Show how each compound can be prepared from an alkene containing 3 carbons (or less).
Each answer will involve the reaction of a Grignard with either a carbonyl or epoxide.
Note: epoxides are prepared from alkenes using a peroxy acid (epoxidation) such as mCPBA.
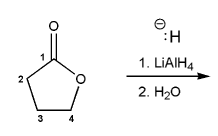
Show a mechanism for the reduction of butyrolactone using LiAlH4.
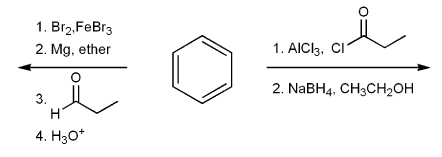
Draw the structure of the major organic product from each reaction sequence.
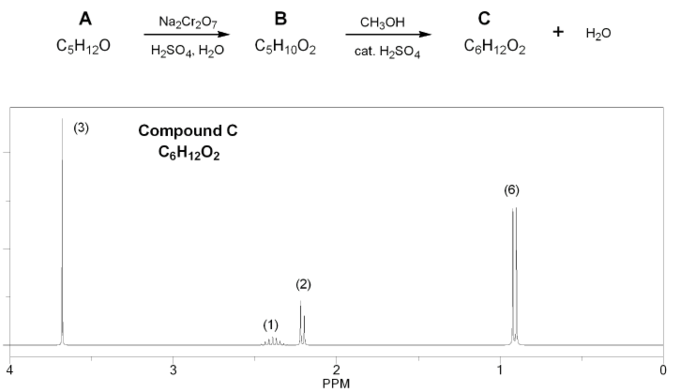
Compound A (C5H12O) is oxidized using aqueous chromium (Jones reagent) to compound B (C5H10O2), which is then treated with methanol under acidic conditions to yield compound C (C6H12O2) and water.
The 1H NMR of compound C is shown below. Determine the structures of compounds A, B, and C.