Let's go over how a carbocation can form from an alkene.
Use curved arrows to show the two carbocations that can from from 1-methylcyclohexene.
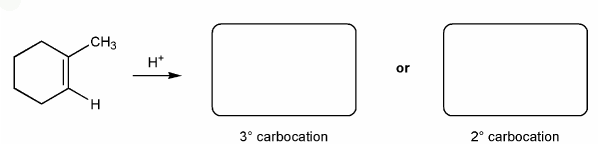
Carbocations aren't very stable and so don't last very long after they are formed.
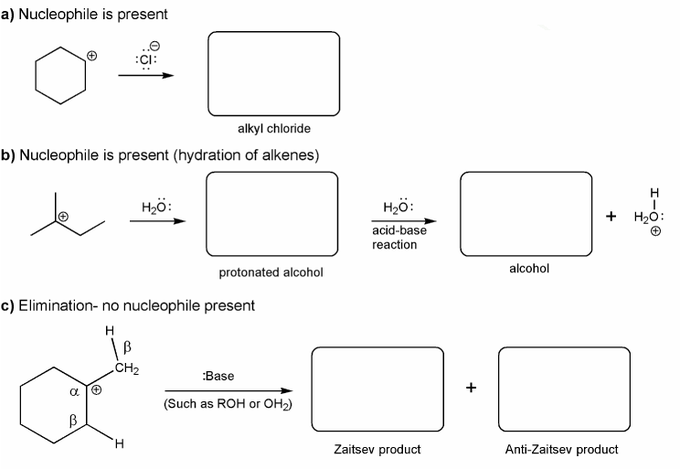
Use curved arrows to show:
a) how a carbocation reacts with a halide ions to form an alkyl halide.
b) how a carbocation reacts with water to form an alcohol.
c) how a carbocation reacts with a base to form an alkene.
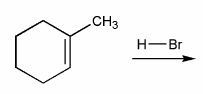
Predict the product(s) of the reaction below, and used curved arrows to show a mechanism.
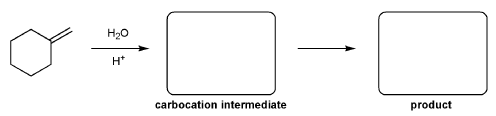
For the reaction below, draw the structures of the carbocation intermediate and the final product.
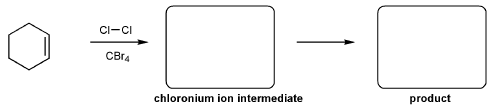
For the reaction below, draw the structures of the chloronium ion intermediate and the final product.
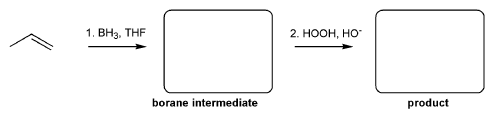
For the reaction below, draw the structures of the borane intermediate and the final product.
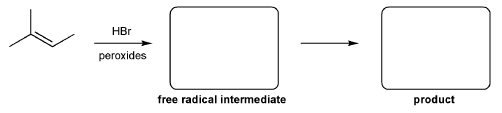
For the reaction below, draw the structures of the radical intermediate and the final product.
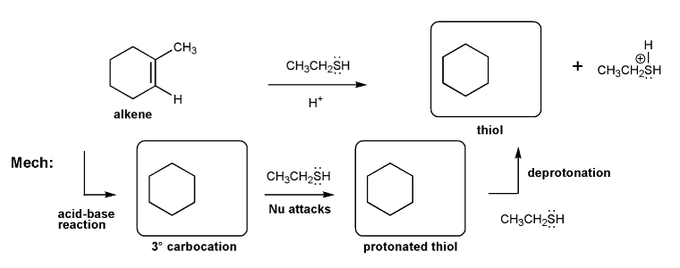
Let's work through an alkene addition reaction. Draw the structures for each of the species in the three boxes below (3º carbocation, protonated thiol, and thiol). Also draw curved arrows to show electron movement. Note: thiol = RSH, like an alcohol, but with sulfur instead of oxygen.
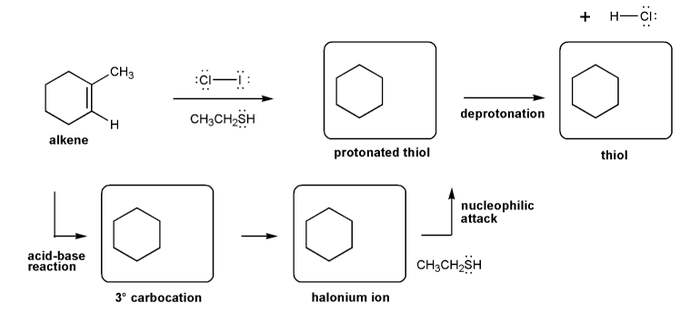
Let's work through a halogenation reaction. Draw the structures for each of the species in the four boxes below (3º carbocation, halonium ion, protonated thiol, and thiol). Also draw curved arrows to show electron movement. Note: thiol = RSH, like an alcohol, but with sulfur instead of oxygen.
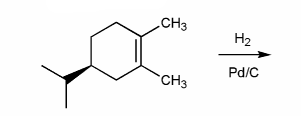
Indicate the major organic product of the reaction below. Include stereochemistry.
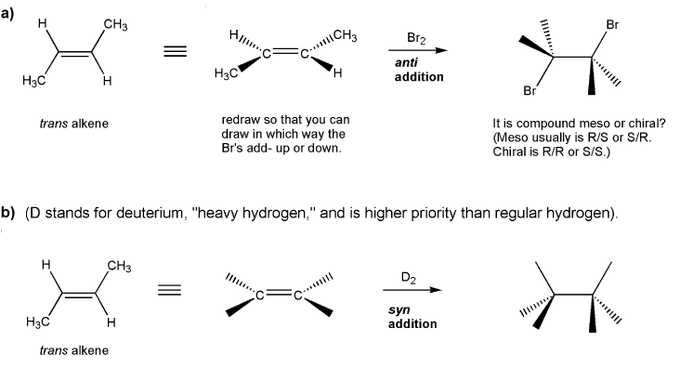
Fill in the product for each reaction below. Indicate stereochemistry where appropriate.
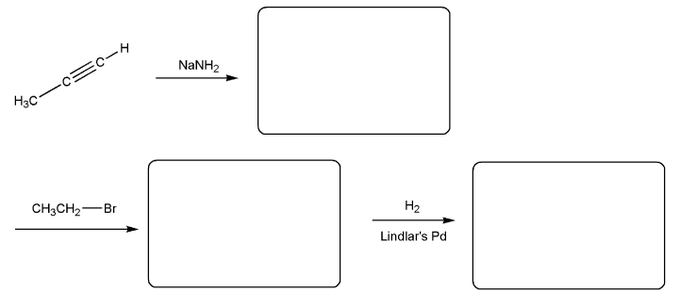
Fill in the product for each reaction below. Indicate stereochemistry where appropriate.
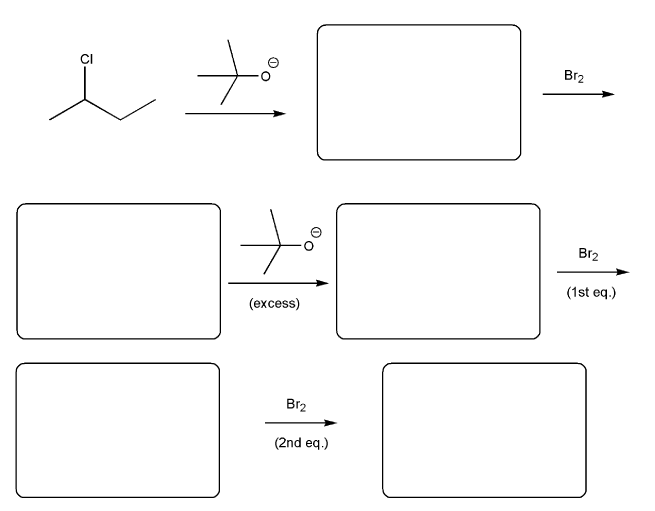
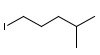
Determine a synthesis to prepare 2-chloro-4-methylpentane from 1-iodo-4-methylpentane.
![]() from
from