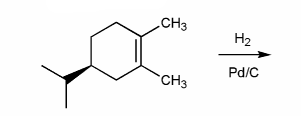
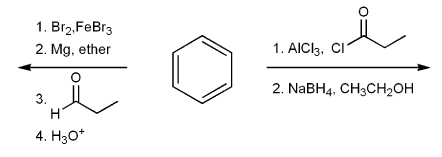
Indicate the major organic product of the reaction below. Include stereochemistry.
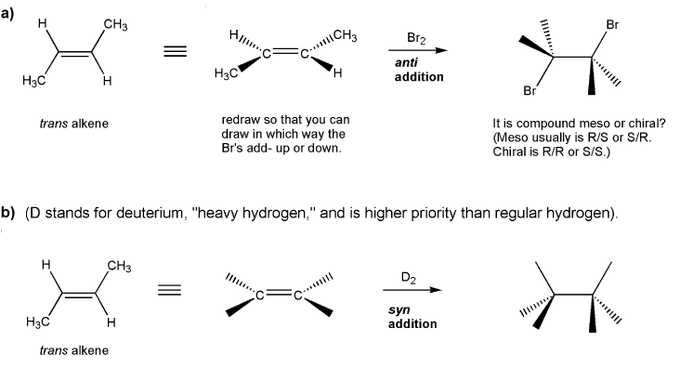
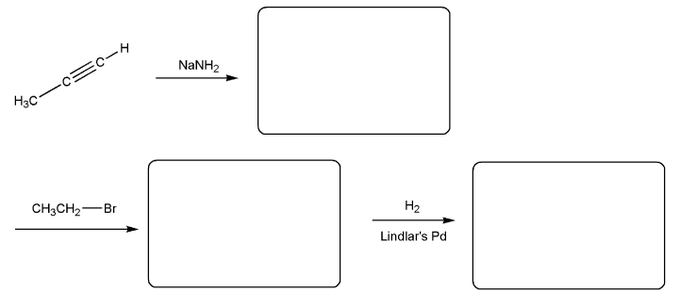
Fill in the product for each reaction below. Indicate stereochemistry where appropriate.
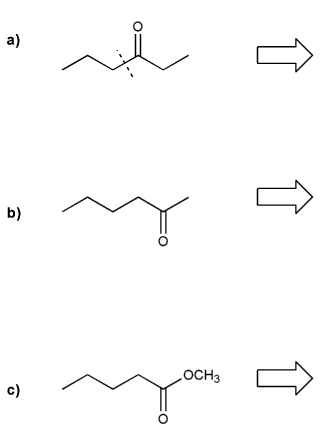
Show how each compound can be prepared from an alkene containing 3 carbons (or less).
Each answer will involve the reaction of a Grignard with either a carbonyl or epoxide.
Note: epoxides are prepared from alkenes using a peroxy acid (epoxidation) such as mCPBA.
Draw the structure of the major organic product from each reaction sequence.
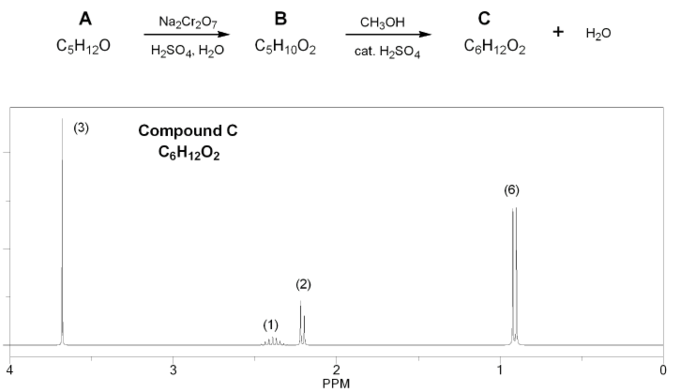
Compound A (C5H12O) is oxidized using aqueous chromium (Jones reagent) to compound B (C5H10O2), which is then treated with methanol under acidic conditions to yield compound C (C6H12O2) and water.
The 1H NMR of compound C is shown below. Determine the structures of compounds A, B, and C.