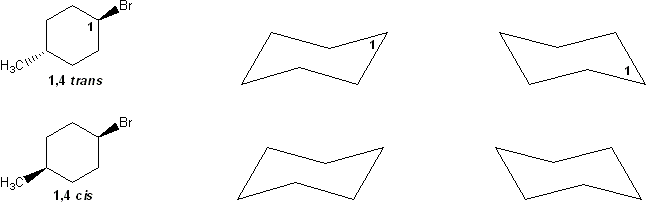
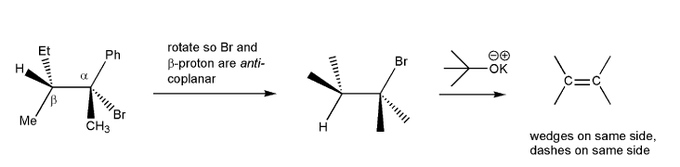
For a molecule to undergo an E2 reaction, the leaving group and the beta-proton must be in an anti-coplanar conformation (one atom straight up, the other straight down). Based on this, which compound undergoes E2 reaction with KOtBu faster? Why?
On the molecule below, mark each stereocenter with an asterisk. (Note: in some textbooks, stereocenters are referred to as stereogenic centers, chirality centers, or asymmetric centers).
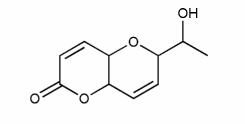
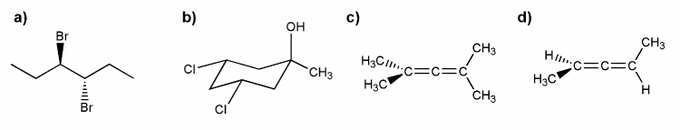
Assign R or S configuration for each molecule below.
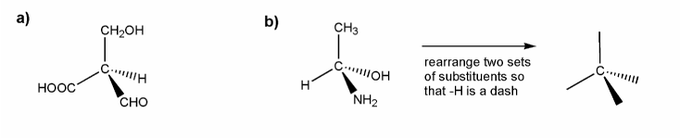
a) is straightforward. I've started you off in b).
Draw the structure of (2R,3S) 2-bromo-3-chlorobutane using wedges and dashes. Also draw a Fischer projection.

Indicate which of the molecules below are chiral (if any).
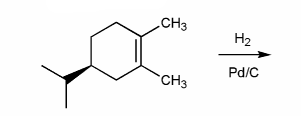
Indicate the major organic product of the reaction below. Include stereochemistry.
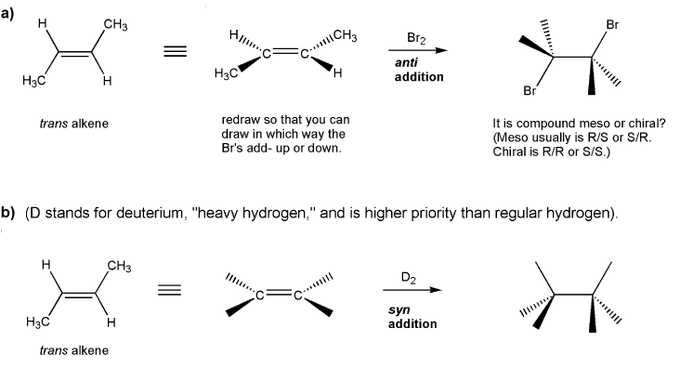
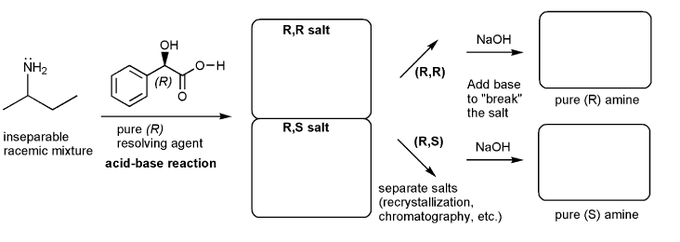
Let's work through a chiral resolution. Write out the structure of the indicated compound in each box. Include stereochemistry.
Why is it possible to separate the (R,R) and (R,S) salts?
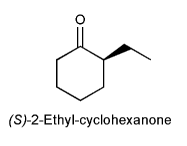
After a sample of optically pure (S)-2-ethyl-cyclohexanone is dissolved in an aqueous solution for several hours, a significant loss of optical activity is observed. Explain.