

Rank the following compounds in order of decreasing boiling point.
Also, make a guess about their relative solubilities in water. Explain your reasoning.
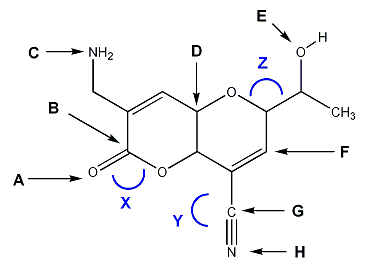
For the molecule shown below, indicate the hybridization (sp3, sp2, sp, etc.) of atoms A through H, and the bond angles of X, Y, and Z.
Rank each set of compounds in order of decreasing boiling point (1 = highest boiling point):
a) ethane, n-octane, n-pentane
b) n-butane, 1-butanol, 1-chlorobutane.
c) n-octane, 2-methylheptane, 2,5-dimethylhexane
(Note that the n- prefix before an alkane just means that it's one chain, without any branching.)